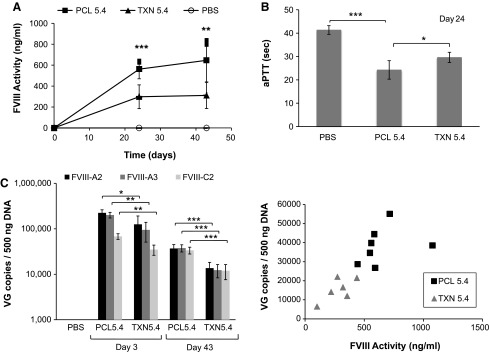
Figure 6.
Comparison of PCL- and TXN-produced rAAVrh8R/FVIII-5.4 vectors in vivo using hemophilia A mice. Vectors were administered via the tail vein at 4 × 1010 vg/mouse. (A) Plasma FVIII protein activity. Activity was measured on days 24 and 43 by a Coatest® assay. (B) Day 24 plasma clotting times measured by an aPTT assay. (C) Vector genome levels in the liver. Vector genome copies on days 3 and 43 were quantified by qPCR using three primer/probe sets (FVIII-A2, FVIII-A3, and FVIII-C2). (D) Correlation of plasma FVIII activity and vector genomes in the liver on day 43. Vector genome levels quantitated by FVIII-A2 primer/probe set are shown. Each point represents one mouse. For all analyses, the method of virus production is indicated (PCL or TXN). Each virus treatment group contained four (day 3) or six to eight (day 43) mice per group (five for the control, PBS, group). Values in each graph represent the average ± standard deviation, and significance was calculated using Student's t-test, with significance indicated as *p < 0.05, **p < 0.01, and ***p < 0.001.