Table 1.
Diastereoselective Oxycyclization and Chelation Studies
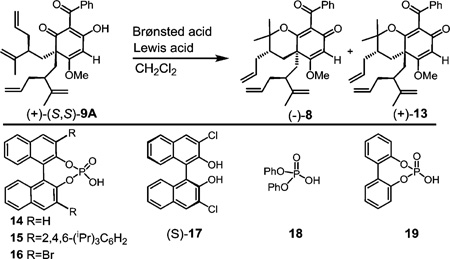 | |||||
|---|---|---|---|---|---|
| entry | Brønsted Acid | Lewis acid | conditions | yield | d.r. (8:13)b |
| 1 | HCla | none | −10 °C to r.t., 72 h | 25%b | 1:1.1 |
| 2 | 19 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 99% | 1:1.8 |
| 3 | 18 (5 equiv) | LiBr (5 equiv) | r.t., 1.5 h | 79% | 1:1.8 |
| 4 | (R)-14 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 91% | 1:2 |
| 5 | (S)-14 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 91% | 1:3 |
| 6 | (R)-15 (5 equiv) | LiBr (5 equiv) | r.t., 48 h | 89% | 1:2.2 |
| 7 | (S)-15 (5 equiv) | LiBr (5 equiv) | r.t., 48 h | 86% | 1:1.8 |
| 8 | (S)-16 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 99% | 1:1.5 |
| 9 | (S)-17 (1 equiv) | SbCl5 (1 equiv) | −78 °C, 2 h | 18% | 9:1 |
| 10 | (S)-14 (5 equiv) | none | −5 to 10 °C, 48 h | 0% | none |
| 11 | none | LiBr (10 equiv) | r.t., 5 d | 19% | 1:1.6 |
| 12 | none | SnCl4 (2 equiv) | −78 to −20 °C, 48 h | 36% | 7:1 |
| 13c | (±)-14 (2 equiv) | SnCl4 (1 equiv) | −78 to 50 °C, 36 h | 58% | 1:1.1 |
| 14 | pTsOH (10 equiv) | BF3OEt2 (79 equiv) | −78 to −5 °C, 1 h | 32%d (57% brsm) | 1:2 |
CH2Cl2/4 M HCl in dioxane (1:1).
Determined by 1H NMR analysis of the crude reaction mixture.
SnCl4 saturated with (±)-14 prior to addition of substrate (+)-(S,S)-9A.
54% starting material also recovered.
