Table 2.
Optimized Lewis/Brønsted Acid Combinations for Diastereoselective Cyclization
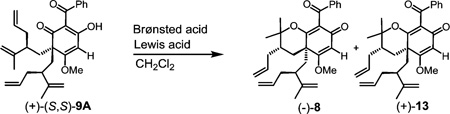 | |||||
|---|---|---|---|---|---|
| entry | Brønsted Acid | Lewis acid | conditions | yield | d.r. (8:13)b |
| 1 | (S)-14 (2 equiv) | SnCl4 (2 equiv) | −78 to −30 °C, 6 d | 68% | 14:1 |
| 2 | (R)-14 (2 equiv) | SnCl4 (2 equiv) | −78 to −30 °C, 6 d | 9% | 1:0 |
| 3a | (±)-14 (2 equiv) | SnCl4 (2 equiv) | −78 to −20 °C, 17 h | 52% | 14:1 |
| 4 | 18 (2 equiv) | SnCl4 (2 equiv) | −78 to 0 °C, 19 h | 47% | 10:1 |
| 5 | 18 (10 equiv) | InCl3 (10 equiv) | r.t., 1 h | 99% | 5.4:1 |
| 6 | pTsOH (10 equiv) | BF3OEt (79 equiv) | −15 to −5 °C, 2 h; −40 to −5 °C, 2 h | 42% | 1:5 |
Substrate (±)-9A was used.
Determined by 1H NMR analysis.
