Abstract
Phylogenetic analyses of DNA sequences from nuclear ribosomal and protein-coding loci support the placement of several perithecial ascomycetes and dematiaceous hyphomycetes from freshwater and terrestrial environments in two monophyletic clades closely related to the Savoryellales. One clade formed by five species of Conioscypha and a second clade containing several genera of uncertain taxonomic status centred on Pleurothecium, represent two distinct taxonomic groups at the ordinal systematic rank. They are proposed as new orders, the Conioscyphales and Pleurotheciales. Several taxonomic novelties are introduced in the Pleurotheciales, i.e. two new genera (Adelosphaeria and Melanotrigonum), three novel species (A. catenata, M. ovale, Phaeoisaria fasciculata) and a new combination (Pleurotheciella uniseptata). A new combination is proposed for Savoryella limnetica in Ascotaiwania s.str. based on molecular data and culture characters. A strongly supported lineage containing a new genus Plagiascoma, species of Bactrodesmiastrum and Ascotaiwania persoonii, was identified as a sister to the Conioscyphales/Pleurotheciales/Savoryellales clade in our multilocus phylogeny. Together, they are nested in a monophyly in the Hypocreomycetidae, significantly supported by Bayesian inference and Maximum Likelihood analyses. Members of this clade share a few morphological characters, such as the absence of stromatic tissue or clypeus, similar anatomies of the 2-layered ascomatal walls, thin-walled unitunicate asci with a distinct, non-amyloid apical annulus, symmetrical, transversely septate ascospores and holoblastic conidiogenesis. They represent the only fungi in the Hypocreomycetidae with apically free, filiform to cylindrical, persistent or partially disintegrating paraphyses. The systematic placement of two other dematiaceous hyphomycetes was resolved based on DNA sequences; Phragmocephala stemphylioides is a member of the Pleurotheciales and Triadelphia uniseptata is within the Savoryellales.
Keywords: freshwater fungi, holoblastic conidiogenesis, Hypocreomycetidae, multigene analysis, Phaeoisaria, systematics
INTRODUCTION
The subclass Hypocreomycetidae (Sordariomycetes) includes non-lichenised ascomycetes with perithecial and cleistothecial ascomata. Many species are parasitic on plants, insects and other fungi. Some are endophytes in plants or saprobes on decaying wood and herbs, and some are involved in obligate mutualism with wood-boring beetles. Based on DNA sequences from nuclear ribosomal and protein-coding loci, the Hypocreomycetidae was recognised as a strongly supported monophyletic clade encompassing five orders (Spatafora et al. 2007, Zhang et al. 2007), i.e. the Coronophorales, Halosphaeriales, Hypocreales, Melanosporales, Microascales, and one family not then placed in an order, the Glomerellaceae. The absence of paraphyses was used to delimit this subclass (Zhang et al. 2007). In the more recent classification, the Hypocreomycetidae comprises eight orders, i.e. the Coronophorales, Falcocladiales, Glomerellales including the Plectosphaerellaceae (Zare et al. 2007, Réblová et al. 2011), Hypocreales, Melanosporales, a revised Microascales (De Beer et al. 2013), Savoryellales (Boonyuen et al. 2011) and Torpedosporales (Schoch et al. 2007, Jones et al. 2014, 2015). Hamathecial elements in the Hypocreomycetidae comprise several types, i.e. apical, centripetal and lateral paraphyses, catenophyses, a reticulate network of filiform filaments attached at the top and bottom of the ascomatal cavity; sometimes interthecial filaments are lacking. The only group characterised by paraphyses, i.e. sterile filiform, apically free filaments emerging from the hymenium among asci and growing upwards, is the Savoryellales, placed in this subclass based on a combined analysis of six nuclear loci (Boonyuen et al. 2011).
The Savoryellales comprises three genera, Ascotaiwania, Canalisporium5 and Savoryella from freshwater, brackish, marine and terrestrial habitats. They share a set of characters including non-stromatic, immersed, semi-immersed to superficial, dark, coriaceous ascomata, often lying horizontally to the host, unitunicate asci with a non-amyloid apical annulus, partly disintegrating paraphyses and fusiform to ellipsoidal, transversely septate ascospores with hyaline polar cells and brown middle cells. Asexual morphs were experimentally proven for two species of Ascotaiwania (as Monotosporella, Ranghoo & Hyde 1998, Sivichai et al. 1998) and one species of Canalisporium (with Ascothailandia sexual morph; Sri-indrasutdhi et al. 2010). The distant placement of Helicoön farinosum, the asexual morph of Ascotaiwania hughesii (Fallah et al. 1999), from members of the Savoryellales was revealed by rDNA data (Boonyuen et al. 2011, Réblová et al. 2012). The asexual morphs linked to the Savoryellales are dematiaceous hyphomycetes characterised by semi-macronematous conidiophores and monoblastic conidiogenous cells producing brown, thick-walled, transversely septate or cheiroid, dictyoseptate macroconidia, rare characters in the Hypocreomycetidae. Although the asexual morphs of Savoryella are unknown (Boonyuen et al. 2011), dark brown, 3–5-septate macroconidia were obtained in living cultures derived from ascospore isolates of two of our specimens of S. limnetica (Chang et al. 1998) collected on wood submerged in freshwater in France. Identical conidia were also observed scattered among ascomata on the host.
Previous phylogenies inferred from sequences of the small and large subunit of nuclear ribosomal DNA (nuc18S and nuc28S rDNA) and the second largest subunit of RNA polymerase II (rpb2) revealed a close relationship among members of the Savoryellales and several terrestrial and freshwater genera of uncertain taxonomic status forming two clades, i.e. Conioscypha and a clade comprising Phaeoisaria, Pleurotheciella, Pleurothecium and Sterigmatobotrys (Réblová et al. 2012). However, relationships among these genera remained largely unresolved. They are characterised by non-stromatic, semi-immersed to superficial, brown, subhyaline to pale orange perithecial ascomata, paraphyses, unitunicate asci with a non-amyloid apical annulus and ellipsoidal to fusiform, hyaline to subhyaline, septate ascospores (Fernández et al. 1999, Réblová & Seifert 2004, 2011, Réblová et al. 2012). Their asexual morphs are hyphomycetes with dematiaceous or hyaline conidiophores, holoblastic, sympodial conidiogenous cells and conidia that are often formed on a short rachis on denticles. The conidiogenesis of Conioscypha is unique; brown, non-septate conidia are born in cyathiform to doliiform blastic conidiogenous cells surrounded by hyaline, cup-like collarettes with a multilamellar structure (Shearer & Motta 1973).
Preliminary analysis of DNA sequences of nuclear ribosomal and protein-coding loci of four undescribed ascomycetes revealed their close relationship with members of the Savoryellales and the clade mentioned above centred around Pleurothecium. Three of these unidentified fungi are perithecial ascomycetes that share with members of the Pleurothecium clade characters of ascomata, asci, paraphyses and ascospores. Five specimens of the first undescribed fungus were found on strongly decaying wood of Quercus cerris in the Czech Republic. Although no conidiophores were formed on the host, cultures derived from ascospore isolates yielded identical asexual morphs with oval to bean-shaped, 1-septate, brown conidia formed holoblastically on a short denticle on almost triangular conidiogenous cells. The second unidentified ascomycete was collected on decaying wood of Fagus sylvatica in the Czech Republic. A single collection of the third undescribed ascomycete was made on decaying wood of Fraxinus excelsior submerged in freshwater in southern France. Cultures of both fungi were derived from isolated ascospores. No conidiophores were observed on the host and none were formed in vitro; only brown, ellipsoidal to globose cells were formed blastically directly on vegetative hyphae in axenic culture. Based on the simple and nondescript sexual morphological characteristics, we could not conclusively attribute any of these three fungi to a known ascomycete genus.
Two morphologically similar specimens of a dematiaceous hyphomycete preliminary identified as Phaeoisaria sp. were made on decaying deciduous wood in Canada and the Czech Republic. They represent a fungus morphologically similar to Ph. clematidis, the type species of the genus, in producing non-septate, obovoid conidia holoblastically on short denticles on sympodially proliferating conidiogenous cells, but differ in the absence of well-developed synnemata on the host and in vitro (Von Höhnel 1909, Deighton 1974). In both strains, the conidiophores were arranged in fascicles and lacked a distinct stipe.
The aim of this study is to investigate phylogenetic relationships of the three unidentified perithecial ascomycetes, Phaeoisaria sp., and also Dactylaria uniseptata and S. limnetica, with members of the Savoryellales and Pleurothecium clade. The affinities of two dematiaceous hyphomycetes Phragmocephala stemphylioides and Triadelphia uniseptata, coincidentally discovered to be related to this clade, are also documented. We also investigate the relationships of taxa characterised by the presence of paraphyses in the subclass Hypocreomycetidae. Although not a part of the presentation of new taxa, our re-examination of this subclass allows further consideration of the Conioscypha clade, presently considered incertae sedis (Réblová & Seifert 2004, Zelski et al. 2014). In order to further clarify the systematic positions of the Conioscypha and Pleurothecium clades, we utilised DNA sequence characters from the nuc rDNA internal transcribed spacer barcode (ITS1-5.8S-ITS2), three protein-coding and two ribosomal nuclear loci.
MATERIALS AND METHODS
Herbarium material and fungal strains
Dry ascomata were rehydrated with water; material was examined with an Olympus SZX12 dissecting microscope and hand-sectioned centrum material (including asci, ascospores and paraphyses) was mounted in Melzer’s reagent, Lugol, 90 % lactic acid, aqueous cotton-blue (1 mg/mL), Pelikan ink and blue or black Waterman ink. Hand sections of the ascomatal wall were studied in 3 % KOH or heated chloral-lactophenol. All measurements were made in Melzer’s reagent. Means ± standard deviation (SD) based on 20–25 measurements are given for dimensions of asci and ascospores. Images were captured by differential interference (DIC) or phase contrast (PC) microscopy using an Olympus DP70 camera operated by Imaging Software Cell on an Olympus BX51 compound microscope. Conidia and conidiogenous cells were photographed in the living state using an FEI Quanta 200 Environmental Scanning Electron Microscope (ESEM). A c. 2 × 2 mm cube of agar with mycelium was observed at 20 kV after the sample chamber achieved local thermodynamic equilibrium: chamber pressure 200 Pa, sample temperature from -15 °C to -16 °C. A Gaseous Secondary Electron Detector (GSED) was used for signal detection. Cooling of the specimen in the chamber was achieved using a PC controlled Peltier cooling stage with external water chiller (made by JT Manufacturing, USA).
Multi-ascospore and multi-conidial isolates were obtained from fresh material with the aid of a spore isolator (Meopta, Prague, Czech Republic). Ascospores and asci were spread on water agar, ascospores and conidia germinated within 48 h. Germinating ascospores were transferred and isolates were grown on water agar, CMA (Difco), potato dextrose agar (PDA, Oxoid) and potato-carrot agar (PCA, Gams et al. 1998). Colonies were examined after 7, 21 and 30 d incubated at 25 °C in the dark. Ex-type and other cultures are maintained at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS) and Canadian Collection of Fungal Cultures, Agriculture and Agri-Food Canada, Ottawa, Canada (DAOMC). Type and other herbarium material is deposited in the Mycological Herbarium in the National Museum in Prague, Czech Republic (PRM) and Canadian National Mycological Herbarium, Ottawa, Canada (DAOM). The Online Auction Colour Chart (2004) was used as the colour standard.
DNA extraction, amplification and sequence alignment
Cultures used for DNA isolations were grown as previously described by Réblová et al. (2011) and DNA was extracted following the protocols of Lee & Taylor (1990). Procedures for amplifying and sequencing the internal transcribed spacer rDNA (ITS rDNA), small and large subunit nuclear ribosomal DNA (nuc18S rDNA, nuc28S rDNA), second largest subunit of RNA polymerase II (rpb2) and DNA replication licensing factor (mcm7) were performed as described in Réblová et al. (2011, 2013). A fragment of the 5’-end of the β-tubulin gene region (exons 3 to 6) was amplified and sequenced using primers Bt2a/benA1and Bt2b (Glass & Donaldson 1995, Geiser et al. 1998). Sequences were edited using Sequencher v. 5.0 (Gene Codes Corp., Ann Arbor, MI, USA).
GenBank accession numbers for newly sequenced taxa and other homologous sequences of members of the Savoryellales and two new orders described in this study retrieved from GenBank are listed in Table 1. For detailed investigation of phylogenetic relationships within the Sordariomycetes, sequences of the three loci nuc28S, nuc18S and rpb2 included in Réblová et al. (2015) were downloaded from GenBank and combined with those generated during the present study.
Table 1.
A list of members of the Conioscyphales, Pleurotheciales, Savoryellales and other fungi, their isolate information and new sequences determined for this study and those retrieved from GenBank. Sequences with GenBank accession numbers in bold were generated for this study. Sequence nuc28S* published in Chew et al. (2010).
| Taxon | Source | ex-type | GenBank accession numbers |
|||||
|---|---|---|---|---|---|---|---|---|
| ITS | nuc28S | nuc18S | RPB2 | MCM7 | TUB2 | |||
| Adelosphaeria catenata | CBS 138679 | T | KT278721 | KT278707 | KT278692 | KT278743 | KT278733 | KT278754 |
| Ascotaiwania lignicola | NIL 00005 | – | HQ446364 | HQ446284 | HQ446419 | – | – | |
| Ascotaiwania limnetica | CBS 126576 | – | – | KT278689 | – | KT278731 | – | |
| CBS 126792 | – | – | KT278690 | – | KT278732 | – | ||
| Ascotaiwania mitriformis | HKUCC 3706 | – | AF132324 | – | – | – | – | |
| Ascotaiwania sawadae | SS 00051 | – | HQ446363 | HQ446283 | HQ446418 | – | – | |
| Ascotaiwania persoonii | A57-14C | T | – | AY094190 | – | – | – | – |
| A57-14C | – | AY590295 | – | – | – | – | ||
| Bactrodesmiastrum obovatum | FMR 6482 | – | FR870266 | – | – | – | – | |
| Bactrodesmiastrum pyriforme | FMR 10747 | – | FR870265 | – | – | – | – | |
| FMR 11931 | – | HE646637 | – | – | – | – | ||
| Brachysporiella setosa | HKUCC 3713 | – | AF132334 | – | – | – | – | |
| Canalisporium caribense | SS 03683 | – | GQ390269 | GQ390254 | – | – | – | |
| Canalisporium elegans | SS 00895 | – | GQ390271 | GQ390256 | HQ446425 | – | – | |
| Canalisporium exiguum | SS 00809 | – | GQ390281 | GQ390266 | HQ446436 | – | – | |
| Canalisporium grenadoideum | BCC 20507 | T | – | GQ390267 | GQ390252 | HQ446420 | – | – |
| Canalisporium pulchrum | SS 03982 | – | GQ390277 | GQ390262 | HQ446431 | – | – | |
| Conioscypha japonica | CBS 387.84 | T | – | AY484514 | JQ437438 | JQ437438 | – | – |
| Conioscypha lignicola | CBS 335.93 | T | – | AY484513 | JQ437439 | JQ429260 | – | – |
| Conioscypha minutispora | CBS 137253 | T | – | KF924559 | – | – | – | – |
| Conioscypha peruviana | ILL 41202 | T | – | KF781539 | – | – | – | – |
| Conioscypha varia | CBS 113653 | – | AY484512 | AY484511 | JQ429261 | – | – | |
| Flammispora bioteca | BCC 13367 | T | – | – | AY722100 | – | – | – |
| Helicoön farinosum | DAOM 241947 | JQ429145 | JQ429230 | – | – | – | – | |
| ILLS 53605 | – | AY094189 | – | – | – | – | ||
| ILLS 53605 | – | AY316357 | – | – | – | – | ||
| Magnisphaera stevemossago | CBS 139776 | – | KT278704 | KT278691 | KT278740 | – | – | |
| Melanotrigonum ovale | CBS 138742 | KT278723 | KT278708 | KT278695 | KT278744 | – | KT278756 | |
| CBS 138743 | T | KT278724 | KT278709 | KT278696 | KT278745 | – | KT278757 | |
| CBS 138744 | KT278725 | KT278710 | KT278697 | KT278746 | – | – | ||
| CBS 138815 | KT278722 | KT278711 | KT278698 | KT278747 | – | KT278755 | ||
| M.R. 3685 | KT278726 | KT278712 | – | KT278748 | – | KT278758 | ||
| Phaeoisaria clematidis | CBS 113340 | EU552148 | – | – | – | – | – | |
| DAOM 226789 | JQ429155 | JQ429231 | JQ429243 | JQ429262 | – | – | ||
| Phaeoisaria fasciculata | CBS 127885 | T | KT278719 | KT278705 | KT278693 | KT278741 | – | KT278752 |
| DAOM 230055 | KT278720 | KT278706 | KT278694 | KT278742 | – | KT278753 | ||
| Phaeoisaria sedimenticola | CGMCC 3.14949 | T | JQ074237 | JQ031561 | – | – | – | – |
| Phaeoisaria sparsa | FMR 11939 | – | HF677185 | – | – | – | – | |
| Phaeoisaria sp. | unknown | – | nuc28S* | – | – | – | – | |
| Phragmocephala stemphylioides | DAOM 673211 | KT278730 | KT278717 | – | – | – | – | |
| Pisorisporium cymbiforme | CBS 127887 | – | – | KT278699 | KT278750 | – | – | |
| CBS 127888 | – | – | KT278700 | KT278751 | – | – | ||
| Plagiascoma frondosum | CBS 139031 | T | – | KT278713 | KT278701 | KT278749 | KT278734 | – |
| Pleurotheciella centenaria | DAOM 229631 | T | JQ429151 | JQ429234 | JQ429246 | JQ429265 | – | – |
| Pleurotheciella rivularia | CBS 125238 | T | JQ429160 | JQ429232 | JQ429244 | JQ429263 | KT278735 | KT278759 |
| CBS 125237 | JQ429161 | JQ429233 | JQ429245 | JQ429264 | KT278736 | KT278760 | ||
| Pleurotheciella uniseptata | DAOM 673210 | T | KT278729 | KT278716 | – | – | – | – |
| Pleurothecium obovoideum | CBS 209.95 | T | EU041784 | EU041841 | – | – | – | – |
| Pleurothecium recurvatum | CBS 101581 | JQ429148 | AF261070 | JQ429248 | JQ429266 | – | – | |
| CBS 138747 | KT278728 | KT278714 | KT278703 | – | – | – | ||
| CBS 138686 | KT278727 | KT278715 | KT278702 | – | KT278737 | – | ||
| CBS 131646 | JQ429150 | JQ429236 | JQ429250 | – | – | – | ||
| CBS 131272 | JQ429149 | JQ429237 | JQ429251 | JQ429268 | – | – | ||
| Pleurothecium semifecundum | CBS 131271 | T | JQ429159 | JQ429240 | JQ429254 | JQ429270 | – | – |
| CBS 131482 | JQ429158 | JQ429239 | JQ429253 | – | – | – | ||
| Savoryella appendiculata | NF 00206 | – | – | HQ446293 | HQ446442 | – | – | |
| Savoryella aquatica | SS 03801 | – | HQ446372 | HQ446290 | HQ446441 | – | – | |
| Savoryella lignicola | NF 00204 | – | HQ446378 | HQ446299 | – | – | – | |
| Savoryella longispora | SAT 00322 | – | HQ446380 | HQ446302 | HQ446450 | – | – | |
| Savoryella paucispora | SAT 00866 | – | HQ446381 | HQ446303 | HQ446451 | – | – | |
| Savoryella verrucosa | SS 00052 | – | HQ446374 | HQ446298 | HQ446445 | – | – | |
| Sterigmatobotrys macrocarpa | PRM 915682 | JQ429153 | GU017317 | JQ429255 | – | KT278739 | KT278762 | |
| DAOM 230059 | JQ429154 | GU017316 | – | JQ429271 | KT278738 | KT278761 | ||
| Sterigmatobotrys uniseptata | FMR 11937 | HF677178 | – | – | – | – | – | |
| Taeniolella rudis | DAOM 229838 | JQ429152 | JQ429241 | JQ429256 | JQ429272 | – | – | |
| Triadelphia uniseptata | DAOMC 250376 | – | KT278718 | – | – | – | – | |
Sequences were manually aligned in BioEdit v. 7.1.8 (Hall 1999). Nuclear ribosomal loci were aligned according to the secondary structure of Saccharomyces cerevisiae to improve the decisions on homologous characters and introduction of gaps (Gutell 1993, Gutell et al. 1993, www.rna.ccbb.utexas.edu). These procedures and alignment of the sequences of protein-coding genes were performed as described in Réblová & Réblová (2013).
The single-locus datasets were examined for topological incongruence among loci (ITS: 26 sequences and 616 characters; β-tubulin: 11 sequences and 500 characters; nuc28S: 126 sequences and 1 947 characters; nuc18S: 104 sequences and 1 792 characters; rpb2 segments 5–7: 77 sequences and 1 216 characters; mcm7: eight sequences and 659 characters). The ITS and β-tubulin loci were generated only for members of the new order Pleurotheciales. Because only a few mcm7 sequences were generated, they were not tested for topological conflicts among clades at familial or ordinal rank in the Sordariomycetes. For each individual partition, 500 bootstrap replicates were generated with RAxML-HPC v. 7.0.3 (Stamatakis et al. 2005, Stamatakis 2006) and compared visually for topological conflict among supported clades in phylogenetic trees. A conflict between two loci was assumed to occur when a clade appeared monophyletic with bootstrap support of ≥ 75 % in one tree, but was not supported as monophyletic in another (Mason-Gamer & Kellogg 1996). Individual, conflict-free alignments were concatenated to combine sequences for two subsequent phylogenetic analyses. The multiple sequence alignments are deposited in TreeBASE (Study no. 18187).
Phylogenetic analyses
Phylogenetic relationships of the unidentified fungi and other ascomycetes were resolved by two combined analyses of ITS, nuc18S, nuc28S, β-tubulin, mcm7 and rpb2 sequences of representatives of the Sordariomycetes. We analysed the whole ITS rDNA barcode, the first 2/3 of the 5’ half of the nuc28S, the entire nuc18S, partial mcm7, exons 3–6 of β-tubulin and segments 5–7 of rpb2. Bases 1–155 of the nuc18S, 1–85 of the nuc28S and 1–58 of the rpb2 alignments at the 5’-end and 1470–1947 of the nuc28S alignment at the 3’-end were excluded from analysis because of incompleteness of the majority of the available sequences. The coding regions (exons) 3, 4, 5 and partly 6 of the β-tubulin with a total length of 291 nucleotides were analysed, non-coding regions were excluded. The combined datasets were partitioned into several subsets of nucleotide sites, i.e. ITS, nuc28S, nuc18S, and first, second and third codon positions of β-tubulin, mcm7 and rpb2. Two members of the Leotiomycetes, Leotia lubrica and Microglossum rufum, were used to root the two multilocus phylogenies.
The program MrModeltest2 v. 2.3 (Nylander 2008) was used to infer the appropriate substitution model that would best fit the model of DNA evolution for each sequence dataset and each partition of the combined datasets. Maximum likelihood (ML) and Bayesian inference (BI) analyses were used to estimate phylogenetic relationships. ML analysis was performed with RAxML-HPC v. 7.0.3 with a GTRCAT model of evolution. Nodal support was determined by non-parametric bootstrapping (BS) with 1 000 replicates.
Bayesian inference analysis was performed in a likelihood framework as implemented in MrBayes v. 3.0b4 to reconstruct phylogenetic trees (Huelsenbeck & Ronquist 2001). For the ITS, nuc18S, nuc28S, and rpb2 dataset, we used for each partition the GTR+G+I substitution model. For β-tubulin we used HKY+G, F81 and SYM+G for the first, second and third codon position, and for mcm7 we used HKY+G, GTR+G and GTR for the first, second and third codon position. Two Bayesian searches were performed using the default parameters. Analyses were run for 10 million generations, with trees sampled every 1 000 generations. Tracer v. 1.6.0. (Rambaut et al. 2013) was used to confirm convergence of trees and burn-in. The first 50 000 trees, which represented the burn-in phase of the analysis, were discarded. The remaining trees were used for calculating posterior probabilities (PP) of recovered branches (Larget & Simon 1999).
PHYLOGENETIC RESULTS
In the first analysis, 134 combined nuc18S, nuc28S and rpb2 sequences were assessed for 120 species in 20 orders in the Sordariomycetes. The alignment had 2 767 distinct alignment patterns (ML analysis). In the ML tree shown in Fig. 1, a strongly supported monophyletic clade was resolved (100 ML BS/1.0 PP) in the Hypocreomycetidae with three nested clades. The Savoryellales (100/1.0) was associated with the Conioscypha clade (100/1.0) including five species and Pleurothecium clade (98/1.0) comprising eight genera and three other undescribed ascomycetes. They represent two new lineages of freshwater and terrestrial fungi and are introduced below as the orders Conioscyphales and Pleurotheciales. A strongly supported monophyletic lineage (100/1.0) containing Ascotaiwania persoonii, two species of the dematiaceous hyphomycetous genus Bactrodesmiastrum and one unidentified ascomycete is positioned as a sister to a clade containing Conioscyphales, Pleurotheciales and Savoryellales. Together they form a robust monophylum (100/1.0) in the Hypocreomycetidae, including Flammispora bioteca (BCC 13367) in a basal position.
Fig. 1.
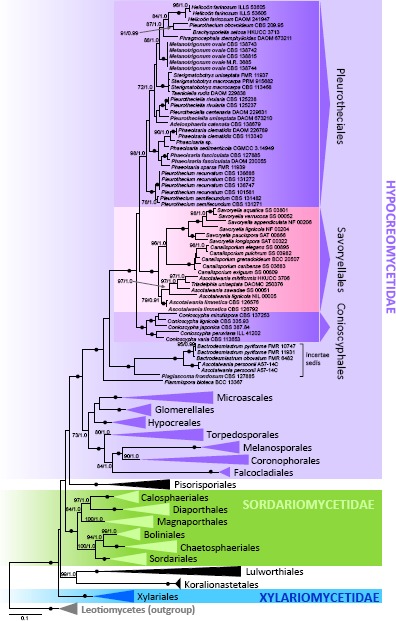
Multilocus phylogenetic analysis of the nuc18S-nuc28S-rpb2 sequences of the Sordariomycetes showing majority of the recognized ordinal lineages. Phylogram was inferred from the ML analysis with RAxML using a GTRCAT model of evolution. Only high branch support is shown at the nodes, maximum likelihood bootstrap support (ML BS) ≥ 70 % and Bayesian posterior probability (PP) ≥ 0.95. Symbol • indicates nodes with 100 % ML BS and 1.0 PP. Taxa written in bold represent taxonomic novelties.
Two undescribed perithecial fungi were nested within the Pleurotheciales and are described here as new genera, Melanotrigonum and Adelosphaeria. Two strains of Phaeoisaria sp. with fasciculate conidiophores were positioned in the strongly supported Phaeoisaria clade (100/1.0) of the Pleurotheciales as the sister taxon to Phaeoisaria sparsa. They are introduced as a new species. The third unidentified perithecial ascomycete from freshwater habitat was nested within the Bactrodesmiastrum clade on a separate branch and it is described as a new monotypic genus Plagiascoma.
Two strains of Savoryella limnetica and Triadelphia uniseptata were positioned in the Ascotaiwania clade (79/0.91) in the Savoryellales. The genus Ascotaiwania is polyphyletic in our phylogeny. Helicoön farinosum, the asexual state of A. hughesii is grouped within the Pleurotheciales. Ascotaiwania lignicola, the type species, and three other species are members of the Savoryellales, while A. persoonii is nested in the Bactrodesmiastrum clade. Two dematiaceous hyphomycetes, Phragmocephala stemphylioides and Dactylaria uniseptata, were grouped among members of the Pleurotheciales; the latter is transferred to Pleurotheciella below.
In the second phylogenetic analysis (Fig. 2), the combined ITS, nuc18S, nuc28S, β-tubulin, mcm7 and rpb2 dataset consisted of 60 sequences representing 18 species of the Pleurotheciales, five of the Conioscyphales, 15 species of the Savoryellales and four species of the Bactrodesmiastrum clade. The alignment had 2 370 distinct alignment patterns (ML analysis). The robust clade containing the three orders and the Bactrodesmiastrum clade (100/1.0) has identical topologies in the three- and six-gene phylogenies. Six terminal clades were identified in the Pleurotheciales and are labelled as Clade I to VI on Fig. 2. Clades I, V and VI are strongly supported monophyletic lineages representing genera Melanotrigonum (100/1.0), Phaeoisaria (100/1.0) and Pleurothecium s.str. (100/1.0). Clade II (72/0.97) is morphologically heterogeneous containing Brachysporiella setosa, Helicoön farinosum, Phragmocephala stemphylioides and Pleurothecium obovoideum. Clade III (100/1.0) includes Sterigmatobotrys and Taeniolella rudis. Clade IV (92/1.0) includes the monophyletic Pleurotheciella (100/1.0) and Adelosphaeria catenata.
Fig. 2.
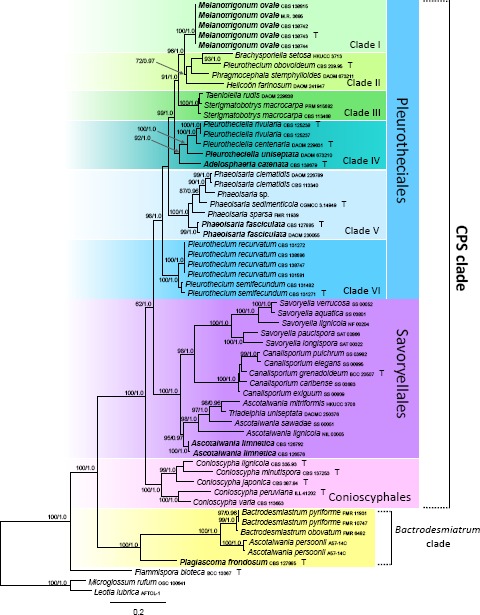
Multilocus phylogenetic analysis of the ITS-nuc18S-nuc28S-β-tubulin-rpb2-mcm7 sequences of the CPS (Conioscyphales, Pleurotheciales, Savoryellales) and Bactrodesmiastrum clades. Phylogram inferred from the ML analysis with RAxML using a GTRCAT model of evolution. Only high branch support is shown at the nodes, ML BS ≥ 70 %, PP ≥ 0.95. Taxa written in bold represent taxonomic novelties.
TAXONOMY
Conioscyphales Réblová & Seifert, ord. nov. — MycoBank MB813226
Type family. Conioscyphaceae Réblová & Seifert.
Ascomata perithecial, non-stromatic. Ostiole periphysate. Hamathecium of paraphyses. Asci unitunicate, with a non-amyloid apical annulus. Ascospores hyaline, transversely multiseptate. Conidiophores micronematous, mononematous. Conidiogenous cells blastic, percurrently regenerating. Conidia brown, variable in shape; secession schizolytic. Saprobic on wood.
Conioscyphaceae Réblová & Seifert, fam. nov. — MycoBank MB813227
Type genus. Conioscypha Höhn., Ann. Mycol. 2: 58. 1904, emend. Shearer, Mycologia 65: 128. 1973.
= Conioscyphascus Réblová & Seifert, Stud. Mycol. 50: 100. 2004.
Ascomata perithecial, immersed to superficial, papillate or with elongated neck. Ascomatal wall leathery, waxy, comprising two layers. Paraphyses filiform, unbranched, longer than the asci. Asci unitunicate, persistent, 8-spored, with a pronounced non-amyloid apical annulus, cylindrical-clavate, stipitate. Ascospores fusiform to fusiform-navicular, hyaline, transversely multiseptate, lacking a mucilaginous sheath or appendages. Conidiophores micronematous, mononematous, hyaline. Conidiogenous cells blastic, cyathiform to doliiform. Conidia brown, non-septate, often with a basal pore, formed singly and successively by percurrent regeneration of the apex of the conidiogenous cell, liberating by apical rupture of the outer wall of the conidiogenous cell.
Pleurotheciales Réblová & Seifert, ord. nov. — MycoBank MB813228
Type family. Pleurotheciaceae Réblová & Seifert.
Ascomata perithecial, non-stromatic. Ostiole periphysate. Hamathecium of paraphyses. Asci unitunicate, with a non-amyloid apical ring. Ascospores hyaline or versicolorous with polar cells hyaline and middle cells brown, transversely multiseptate. Conidiophores macronematous or semi-macronematous, loosely fasciculate or aggregated in indeterminate synnemata. Conidiogenous cells producing conidia holoblastically, monoblastic or with sympodial extension, conidial secession rhexolytic or schizolytic. Conidia hyaline or brown or versicolorous, septate or non-septate. Saprobic on wood, rarely human pathogens causing keratomycosis.
Pleurotheciaceae Réblová & Seifert, fam. nov. — MycoBank MB 813229
Type genus. Pleurothecium Höhn., Ber. Deutsch. Bot. Ges. 37: 154. 1919.
= Carpoligna F.A. Fernández & Huhndorf, Mycologia 9: 253. 1999.
Ascomata perithecial, immersed to semi-immersed to superficial, papillate or with a central rarely eccentric neck. Ostiole periphysate. Ascomatal wall leathery to fragile, carbonaceous, brown, comprising two layers. Paraphyses abundant, sparsely branched, partially disintegrating, cylindrical. Asci unitunicate, 8-spored, with a pronounced non-amyloid apical annulus, cylindrical or cylindrical-clavate. Ascospores ellipsoidal to fusiform, hyaline or versicolorous with polar cells hyaline and middle cells brown, transversely multiseptate, lacking a mucilaginous sheath or appendages. Conidiomata present or absent, when present indeterminate synnemata or loose fascicles. Conidiophores macronematous or semi-macronematous, sometimes regenerating percurrently. Conidiogenous cells producing conidia holoblastically, conidial secession rhexolytic on short denticles or rachis on sympodially extending polyblastic conidiogenous cells, or schizolytic on monoblastic or solitary thallic conidiogenous cells. Conidia hyaline, sometimes with protracted maturation of the middle cells, which turn brown, or brown or versicolorous, septate or non-septate.
Adelosphaeria Réblová, gen. nov. — MycoBank MB813230
Type species. Adelosphaeria catenata Réblová.
Etymology. Adelo- (Gk), meaning unclear, referring to the difficulty of recognising this taxon among other morphologically similar fungi; sphaera (L) meaning globe, referring to ascoma.
Ascomata perithecial, non-stromatic, semi-immersed becoming superficial, subglobose, dark brown, papillate. Ostiole periphysate. Ascomatal wall leathery to fragile, 2-layered. Paraphyses abundant, persistent, septate. Asci unitunicate, cylindrical-clavate, stipitate, 8-spored, apex with a non-amyloid apical annulus. Ascospores ellipsoidal, slightly curved, hyaline, transversely septate. Asexual morph unknown.
Adelosphaeria catenata Réblová, sp. nov. — MycoBank MB813231; Fig. 3, 4
Fig. 3.
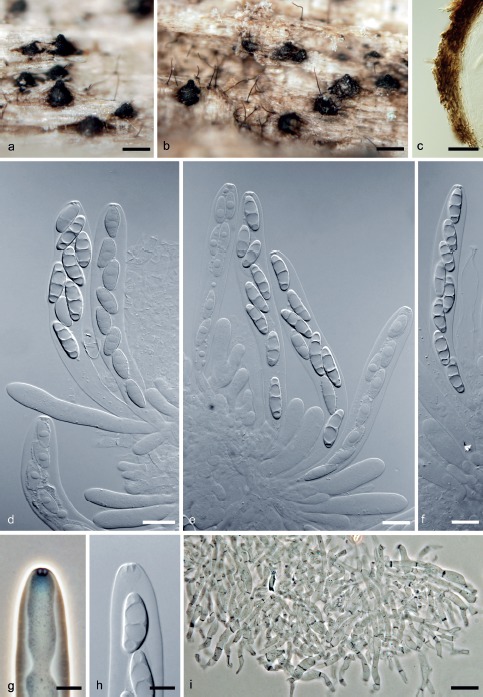
Adelosphaeria catenata. a, b. Ascomata; c. vertical section of the ascomatal wall; d–f. asci with ascospores; g, h. apical annulus; i. paraphyses (a–i. PRM 933853 holotype); d–f, h: DIC; g, i: PC. — Scale bars: a, b = 250 μm; c = 30 μm; d–f, i = 10 μm; g, h = 5 μm.
Fig. 4.
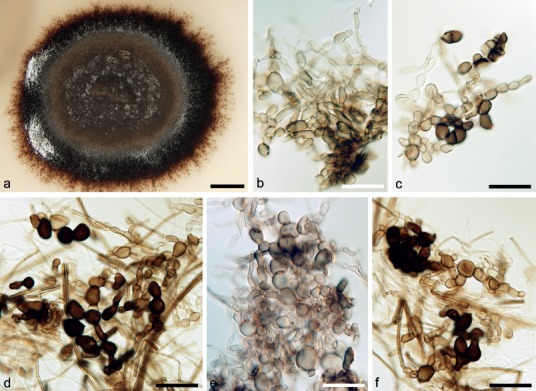
Asexual morph of Adelosphaeria catenata. a. Colony on PCA; b–f. brown cells arising blastically from vegetative mycelium on PCA (a–f. CBS 138679, 21 d, 25 °C); b–f: DIC. — Scale bars: a = 4 mm; b–f = 20 μm.
Etymology. Cateniformis (L), meaning chain-shaped, referring to the dark brown cells arranged in chains formed on vegetative hyphae in the axenic culture.
Ascomata perithecial, non-stromatic, semi-immersed, becoming superficial, solitary or in small groups; venter 200–280 μm diam, 300–360 μm high, subglobose, dark brown, glabrous, papillate, opening by a rounded pore. Ostiole periphysate. Ascomatal wall leathery to fragile, 20–30 μm thick, 2-layered; outer layer consisting of brown, polyhedral cells of textura prismatica with opaque walls, inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, persistent, septate, hyaline, sparsely branched, anastomosing, c. 3.5–5.0 μm wide, tapering to c. 2.5 μm. Asci (85–)93–105 μm long in the sporiferous part, 12.5–14.5 μm wide (mean ± SD = 199.7 ± 5.4 × 12.5 ± 1.2 μm), with a stipe 20–35(–50) μm long; cylindrical-clavate, broadly rounded apically to obtuse, 8-spored, apex with a flattened, non-amyloid apical annulus 3.0–3.5 μm wide, about 2.0 μm high. Ascospores 16.5–19.5(–20) × 5.0–5.5(–5.8) μm (mean ± SD = 17.8 ± 1.3 × 5.4 ± 0.2 μm), ellipsoidal, straight or slightly curved, hyaline, smooth, 3-septate, non-constricted at the septa, arranged 1–2-seriately in the sporiferous part.
Culture characteristics — Colonies slow growing reaching 12–15 mm diam on PDA after 21 d at 25 °C. Aerial mycelium dark brown (aoc735), paler brown (aoc723) towards the margin, mainly flat, felty, reverse brown (aoc734), with a marginal zone of dark brown (aoc734) submerged mycelium. Aerial and submerged hyphae 1.5–2.0 μm wide, smooth, subhyaline to pale brown, thin-walled, unbranched or sparsely branched. Sporulation absent. On vegetative hyphae are formed brown, globose to ellipsoidal cells 5.0–14.5 μm diam (mean ± SD = 10.3 ± 2.9 μm), with thick, often opaque walls, arranged in chains or arising laterally on another cell.
Specimen examined. CZECH REPUBLIC, Southern Bohemia, Novohradské hory Mts, Dobrá voda, Hojná voda National nature monument, decorticated wood of a trunk of Fagus sylvatica, 4 Oct. 2012, M. Réblová M.R. 3755 (holotype PRM 933853, culture ex-type CBS 138679).
Notes — Adelosphaeria catenata resembles species of Pleurothecium and Pleurotheciella because of its hyaline, 3-septate ascospores, cylindrical-clavate asci and brown semi-immersed ascomata. Pleurothecium recurvatum is easily distinguishable from A. catenata by its slender ascospores, pronounced apical annulus, setose ascomata and macronematous conidiophores bearing hyaline, polyblastic, denticulate conidiogenous cells elongating in a sympodial pattern and 3-septate, hyaline conidia with protracted maturation of the middle cells, which turn brown. It is more difficult to separate Adelosphaeria from Pleurotheciella, because species of both genera share similar morphological characters of ascospores, asci and ascomata. The only conspicuous difference lies in morphology of their asexual states; Pleurotheciella can be easily distinguished by Dactylaria-like, hyaline to subhyaline conidiophores and conidia.
Melanotrigonum Réblová, gen. nov. — MycoBank MB813232
Type species. Melanotrigonum ovale Réblová.
Etymology. Melas- (Gk), meaning dark, referring to the brown conidia; Trigonon (Gk) meaning triangle, referring to conspicuous triangle-like conidiogenous cells of the asexual morph.
Ascomata perithecial, non-stromatic, immersed to semi-immersed, subglobose to broadly conical, dark brown, papillate. Ostiole periphysate. Ascomatal wall leathery to fragile, 2-layered. Paraphyses abundant, persistent, septate. Asci unitunicate, cylindrical, stipitate, 8-spored, apex with a conspicuous, non-amyloid apical annulus. Ascospores ellipsoidal, hyaline, transversely septate. Asexual morph a dematiaceous hyphomycete, conidiophores semi-micronematous, conidiogenous cells producing brown, 1-septate conidia holoblastically on short denticles.
Melanotrigonum ovale Réblová, sp. nov. — MycoBank MB813233; Fig. 5, 6
Fig. 5.
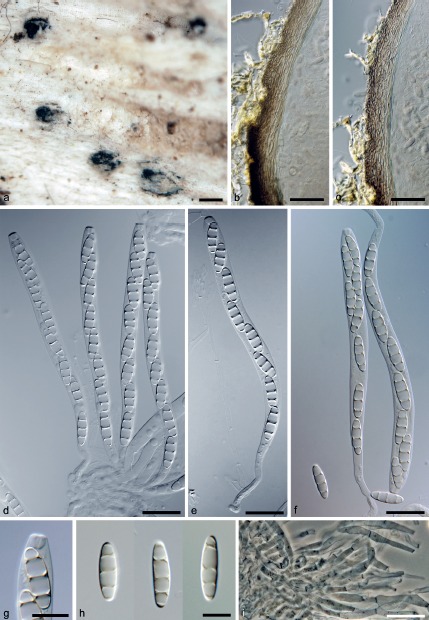
Melanotrigonum ovale. a. Ascomata; b, c. vertical sections of the ascomatal wall; d–f. asci with ascospores; g. apical annulus; h. ascospores; i. paraphyses (a–i. PRM 933852 holotype); b–h: DIC; i: PC. — Scale bars: a = 250 μm; b, c = 25 μm; d–f, i = 20 μm; g, h = 10 μm.
Fig. 6.
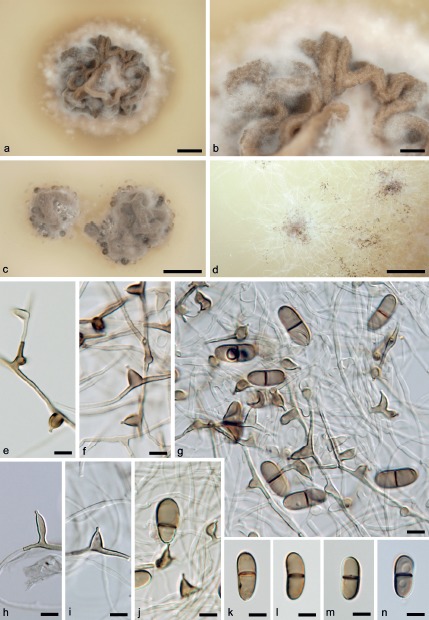
Asexual morph of Melanotrigonum ovale. a, b. Colony on PDA (5 mo, 25 °C); c, d. colony on PDA (1 mo, 25 °C); e, f, h, i. conidiogenous cells on PCA; g, j. conidiogenous cells with conidia borne on a denticle on PCA; k–n. conidia (a, b, g–i, k–n. M.R. 3685; c, d–f, j. CBS 138742; e–j. 21 d, 25 °C); e–n: DIC. — Scale bars: a = 2.5 mm; b = 5 mm; c, d = 10 mm; e–n = 5 μm.
Etymology. Ovalis (L), referring to the oval shape of conidia.
Ascomata perithecial, non-stromatic, immersed to semi-immersed, gregarious, occurring in small to large groups; venter 320–480 μm diam, 400–500 μm high, subglobose to broadly conical, brown, glabrous, papillate, opening by a rounded pore. Ostiole periphysate. Ascomatal wall leathery to fragile, 23–30 μm thick, 2-layered; outer layer consisting of brown, polyhedral cells of textura prismatica with opaque walls; towards the exterior grading into polyhedral to angular cells of textura angularis; towards the interior grading into pale-brown, elongated cells. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, persistent, septate, anastomosing, hyaline, sparsely branched, c. 3.0–4.5 μm wide, tapering to c. 3.0 μm, longer than the asci. Asci (105–)115–128(–142) μm long in the sporiferous part, (8.5–)9.0–11.5 μm wide (mean ± SD = 122.8 ± 7.4 × 11.0 ± 5.2 μm) with a stipe 32–50 μm long; cylindrical, obtuse apically, 8-spored, apex with a large, conspicuous non-amyloid apical annulus 4.5–5.0 μm wide, 3.5–4.0 μm high. Ascospores (17–)18–21(–21.5) × 5.0–6.0(–6.5) μm (mean ± SD = 19.4 ± 1.5 × 5.8 ± 0.3 μm), ellipsoidal, straight to slightly curved, hyaline, smooth, 3-septate, non-constricted at the septa, arranged obliquely uniseriate, sometimes 2-seriate only in the upper part of the ascus.
Culture characteristics — Colonies slow growing, reaching 8–10 mm diam on PDA after 21 d at 25 °C. Aerial mycelium beige in the centre of the colony and on the inoculum block, white towards the margin, felty, centre elevated, later with a moist appearance, bent into deep folds, reverse dark beige. Aerial hyphae 2.0–3.0 μm wide, smooth, hyaline, thin-walled, sparsely branched. Submerged hyphae 2.0–2.5 μm wide, smooth, hyaline. Sporulation appears later on the youngest aerial hyphae at the margin of the colony. Conidiophores semi-micronematous, reduced to a conidiogenous cell, arising vertically from hyphae, unbranched, smooth, tapering towards the apex. Conidiogenous cell 4.5–8.0(–10.0) μm long, 2.0–3.0 μm wide in the broadest point (mean ± SD = 6.9 ± 1.1 × 2.8 ± 0.4 μm), integrated, intercalary, almost triangular to ampulliform, tapering towards the apex, pale brown, with a single, rarely two, pale brown to subhyaline denticle 1.0–2.0 μm long. Conidia (10–)11.5–13.5(–14) × 5.0–6.0 μm (mean ± SD = 12.3 ± 0.6 × 5.4 ± 0.5 μm), 1-septate, oval to bean-shaped, straight or slightly curved, leaving a pore when detached, smooth, rounded at both ends or slightly tapering towards the base, brown, darker at the septum, non-constricted, sometimes cells asymmetrical.
Specimens examined. CZECH REPUBLIC, Southern Moravia, Břeclav distr., Valtice, Rendezvous Valtice National nature monument, decaying wood of a trunk of Quercus cerris, 17 Nov. 2012, M. Réblová M.R. 3698 (holotype PRM 933852, culture ex-type CBS 138743); ibid., M.R. 3685, M.R. 3688A (culture CBS 138742), M.R. 3688B (culture CBS 138744), M.R. 3699 (culture CBS 138815).
Notes — Five strains of M. ovale were collected on soft, strongly decaying wood of several fallen trunks of Quercus cerris, the remains of old growth trees that were more than hundred years old. Specimen M.R. 3685 has asci shorter in the sporiferous part (100–105 μm long) and generally smaller ascospores, (15–)16–17.5(–19.5) × 4.5–5.5 μm.
Melanotrigonum ovale is similar to Pleurotheciella rivularia in characters of ascospores, asci and ascomata, but differs from it by the ascal apex with a conspicuous and larger apical annulus, 4.5–5.0 × 3.5–4.0 μm (w × h) vs 2.5–3.5 × 1.5 μm in P. rivularia. Both species produce conidia on denticulate conidiogenous cells. In Melanotrigonum, the conidiogenous cells are almost triangular to ampulliform, tapering towards the apex with a single or rarely two denticles, while conidiogenous cells of Pleurotheciella are cylindrical, elongate sympodially with one to several denticles.
Plagiascoma Réblová & J. Fourn., gen. nov. — MycoBank MB813234
Type species. Plagiascoma frondosum Réblová & J. Fourn.
Etymology. Plágios (Gk), meaning slanting, oblique, sideways, referring to the flattened ascomata arranged horizontally to the host.
Ascomata perithecial, non-stromatic, immersed gradually erumpent to semi-immersed, conical, dark brown, lying obliquely to horizontally, papillate or with a neck. Ostiole periphysate. Ascomatal wall fragile, 2-layered. Paraphyses abundant, persistent, septate. Asci unitunicate, cylindrical to cylindrical-fusiform, stipitate, 8-spored, apex with a non-amyloid apical annulus. Ascospores fusiform, hyaline, transversely septate. Asexual morph unknown.
Plagiascoma frondosum Réblová & J. Fourn., sp. nov. — MycoBank MB813235; Fig. 7
Fig. 7.
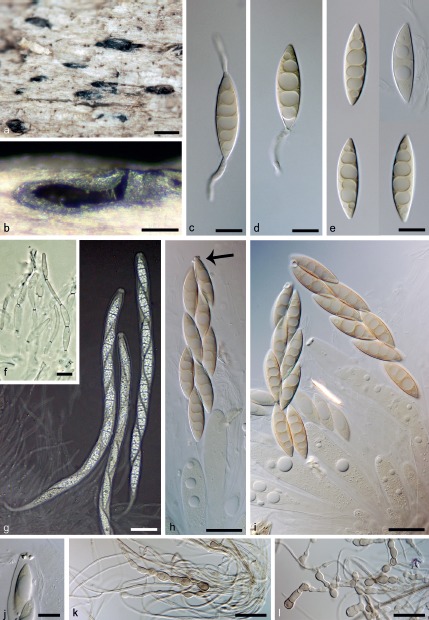
Plagiascoma frondosum. a. Ascomata; b. vertical section of the ascomatal wall; c, d. germinating ascospores; e. ascospores; f. paraphyses; g. asci in freshly collected material in Pelikan ink; h, i. asci from air dried herbarium material, arrow indicates apical annulus; j. apical annulus; k, l. pigmented cells formed in vitro on vegetative hyphae on PCA (a–j. PRM 933854 holotype; k, l. CBS 139031, 21 d, 25 °C); c–e, h–l: DIC; f, g: PC. — Scale bars: a = 500 μm; b = 250 μm; c–f, j = 10 μm; g = 25 μm; h, i, k, l = 20 μm.
Etymology. Frondosus (L), meaning leaf-bearing, referring to a deciduous tree as a host.
Ascomata non-stromatic, immersed, gradually erumpent to semi-immersed, solitary or in small groups or in rows; venter 200–280 μm diam, 450–550 μm high, conical, dark brown, glabrous, slightly pinched laterally, lying obliquely to horizontally, papillate or with a beak 30–120 μm high, conical, lateral, opening by a rounded pore. Ostiole periphysate. Ascomatal wall fragile, 24–30 μm thick, becoming thicker in the beak up to c. 35 μm, 2-layered; outer layer consisting of brown, polyhedral, flattened cells of textura prismatica with opaque walls. Inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Paraphyses abundant, persistent, septate, hyaline, c. 4.0–6.0(–7.0) μm wide, tapering to c. 3.5 μm. Asci in fresh material 225–240 μm long in the sporiferous part, 13–15 μm wide, with a stipe 30–52 μm long, cylindrical, with ascospores arranged obliquely uniseriate; upon drying asci 100–160 μm long in the sporiferous part, 15–20 μm wide with a stipe 53–73 μm long, cylindrical-fusiform, with ascospores arranged 2-seriately; obtuse to broadly rounded apically, 8-spored; apex with a non-amyloid apical annulus 4.5–5.5 μm wide, 1.5–2.5 μm high. Ascospores (28.5–)30–34.5(–36) × 7.5–8.5(–9.0) μm (mean ± SD = 32.8 ± 1.9 × 8.4 ± 0.6 μm), fusiform, tapering towards the ends, hyaline, smooth, 3–5-septate, non-constricted at the septa, with a large guttule in each cell.
Culture characteristics — Colonies slow growing, 10–15 mm diam on PDA after 21 d at 25 °C. Aerial mycelium brown near the centre of the colony and on the inoculum block, pale brown to beige (oac800) towards the margin, felty, reverse brown (oac734). Aerial and submerged hyphae 1.5–2.5 μm wide, smooth, subhyaline, thin-walled, sparsely branched. Sporulation absent. On aerial hyphae arise ellipsoidal cells 5.5–9.0 μm diam (mean ± SD = 7.0 ± 1.0 μm), pale brown to subhyaline, thick-walled, intercalar, terminal or arranged in a short chain.
Specimen examined. FRANCE, Midi-Pyrénées, Ariège, Rimont, valley of La Maille brook, c. 550 m asl, submerged decorticated wood of Fraxinus excelsior, 9 May 2014, J. Fournier J.F. 14044 (holotype PRM 933854, culture ex-type CBS 139031).
Notes — The examination of fresh material of P. frondosum revealed asci over 200 μm long and 13–15 μm wide, with uniseriate ascospores arranged obliquely (Fig. 7g). Upon drying, the arrangement of ascospores changes and they became biseriate within the ascus. The asci in dry herbarium material are shorter in the sporiferous part, 100–160 μm long, and wider 15–20 μm with almost twice the stipe length (Fig. 7h, i). No sheath or appendages were observed on immature or mature ascospores. Freshwater perithecial ascomycetes often have ascospores enclosed in a hyaline sheath or have appendages to facilitate their attachment on moist woody substrates. Interestingly, this is largely true for species from Asia, America and Australia but not in Europe, where many of the most widespread freshwater species lack these structures.
The fusiform, hyaline, 3–5-septate ascospores of P. frondosum resemble multiseptate ascospores of some species of Annulatascus, e.g. A. nilensis (Abdel-Wahab et al. 2011) and A. tropicalis (Tsui et al. 2002). In our multilocus phylogeny, P. frondosum is positioned in the strongly supported Bactrodesmiastrum clade.
Phaeoisaria fasciculata Réblová & Seifert, sp. nov. — MycoBank MB813236; Fig. 8
Fig. 8.

Phaeoisaria fasciculata. a. Colony on PCA; b. conidiophores in vivo; c. conidia in vivo; d, f, g–i. conidiophores on PCA; e. conidia on PCA (a, d–f. CBS 127885; b, c. PRM 933855 holotype; g–i. DAOM 230055; a, d–i. 21 d, 25 °C); b–f: DIC; g–i: ESEM. — Scale bars: a = 50 μm; b = 250 μm; b, d, f = 20 μm; c, e = 10 μm; g–i = 10 μm.
Etymology. Fasciculus (L), meaning fascicle or bundle, referring to conidiophores arranged in fascicles and lacking a distinct stipe.
Colonies in vivo effuse, dark grey, whitish to beige when sporulating. Sexual morph not observed. Synnemata absent, conidiophores forming fascicles. Conidiophores 25–65 × 3.0–3.5 μm (mean ± SD = 41.7 ± 14.2 × 3.3 ± 0.3 μm), macronematous, arising from brown, thick-walled cells, cylindrical, pale brown, subhyaline towards the apex, unbranched, smooth-walled. Conidiogenous cells 10–29(–36) × 2.5–3.5 μm (mean ± SD = 20.2 ± 6.7 × 3.1 ± 0.5 μm), integrated, terminal, cylindrical, tapering towards tip, pale brown to subhyaline near base, hyaline towards apex, smooth-walled, polyblastic, forming conidia sympodially on conspicuous denticles 1.0–1.5 μm long, about 0.5 μm wide, scattered or clustered in the apical region. Conidia 6.0–8.0(–9.0) μm long, about 2.0 μm wide (mean ± SD = 7.3 ± 1.2 × 2.0 ± 0.1 μm), ellipsoidal to obovoid, straight, rounded at the apex, obtuse and tapering towards base, hyaline, non-septate, smooth-walled.
Culture characteristics — Colonies reaching 12–18 mm diam on PCA after 21 d at 25 °C. Aerial mycelium beige to pale brown (oac662), at first smooth, later cottony, reverse brown (oac640). Aerial and submerged hyphae 2.0–3.0 μm wide, hyaline to pale brown, sparsely branched, smooth-walled. Sporulation appears first in the centre of the colony, later present over the whole colony or in isolated patches; sporulating colony beige (oac809) with a powdery appearance. Synnemata absent. Conidiophores 20–75 × 2.5–3.5 μm (mean ± SD = 45.3 ± 17.0 × 3.0 ± 0.3 μm), macronematous to semi-macronematous reduced to a single conidiogenous cell, arising from aerial hyphae, cylindrical, slightly tapering towards the apex, pale brown, subhyaline towards the apex, unbranched, smooth-walled. Conidiogenous cell 10–29(–36) × 2.5–3.5(–4.0) μm (mean ± SD = 20.2 ± 6.7 × 3.1 ± 0.5 μm), integrated, terminal and intercalar, cylindrical, tapering towards tip, pale brown to subhyaline, hyaline towards apex, smooth-walled, polyblastic, with numerous conspicuous denticles 1.0–1.5 μm long, c. 0.5 μm wide, scattered along the whole length of intercalar conidiogenous cell and clustered in the apical region. Conidia 5.5–7.5(–8.5) × 2.0–2.5(–3.0) μm (mean ± SD = 6.7 ± 0.9 × 2.5 ± 0.3 μm), ellipsoidal to obovoid, straight, rounded at the apex, obtuse and tapering towards base, hyaline, non-septate, smooth-walled.
Specimens examined. CANADA, Ontario, Goulbourn Twp., Stittsville, bark on branch on ground, 8 Oct. 2001, Keith A. Seifert K.A.S. 1433 (DAOM 230055). – CZECH REPUBLIC, Southern Moravia, Břeclav distr., Milovice, Milovická stráň Nature Reserve, north slopes of Mt Špičák, 293 m asl, decorticated wood of Sambucus nigra, 18 Nov. 2009, M. Réblová M.R. 3084 (holotype PRM 933855, culture ex-type CBS 127885).
Notes — Phaeoisaria fasciculata is easily distinguished from other species of the genus by its conidiophores, which grow in fascicles on the host, while typical indeterminate synnemata are not formed. The ellipsoidal to obovoid, non-septate conidia of Ph. fasciculata resemble those of Ph. caffera, the Ph. clematidis species complex and Ph. magnifica. Phaeoisaria caffera differs from the new species by longer, pale yellowish brown conidia. The conidia of Ph. clematidis and Ph. magnifica are subhyaline to very dilute olivaceous and generally wider.
Ascotaiwania limnetica (H.S. Chang & S.Y. Hsieh) Réblová & J. Fourn., comb. nov. — MycoBank MB813237; Fig. 9, 10
Fig. 9.
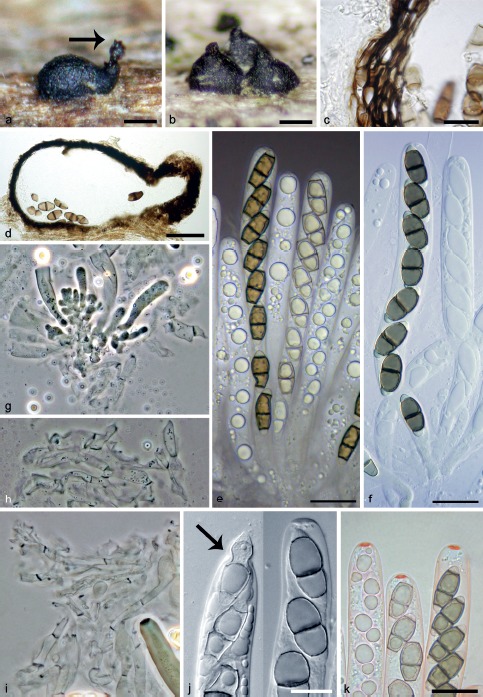
Ascotaiwania limnetica. a, b. Ascomata, arrow indicates ascospores aggregated at the top of the neck; c, d. vertical sections of the ascomatal wall; e. asci with ascospores in Pelikan ink; f. asci with ascospores; g–i. paraphyses; j. apical annulus, arrow indicates the tip of ascal apex, when ascospore is released through the annulus; k. asci with apical annulus in Congo red (a–e, k. PRM 933850; f, g, i, j. PRM 933851; h. PRM 933849); c–f, j, k: DIC; g–i: PC. — Scale bars: a, b = 150 μm; c, j = 10 μm; d = 100 μm; c, e = 10 μm; e, f, k = 50 μm.
Fig. 10.
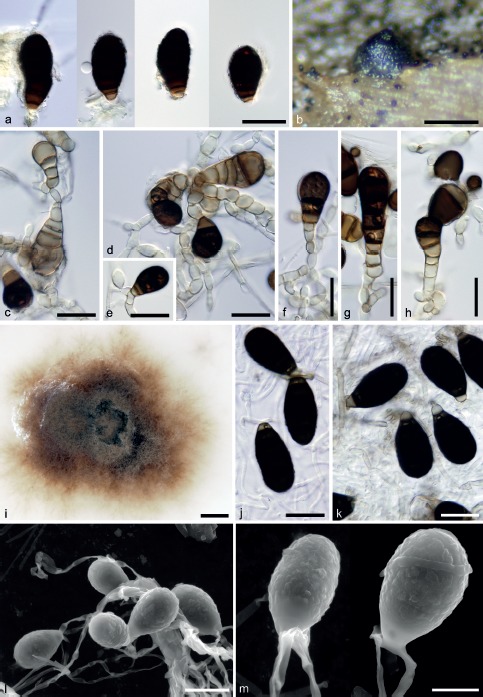
Asexual morph of Ascotaiwania limnetica. a. Conidia in vivo; b. ascoma with macroconidia scattered on wood surface; c–h. conidia and conidiogenous cells on PDA; i. colony on PCA; j–m. conidia on PCA (a, c–h. CBS 126576; b. PRM 933850; j–m. CBS 126792; a, c–m. 21 d, 25 °C); a, c–h, j, k: DIC; l, m: ESEM. — Scale bars: a, c–h, j, k, l = 20 μm; b = 250 μm; i = 5 mm; m = 10 μm.
Basionym. Savoryella limnetica H.S. Chang & S.Y. Hsieh, Mycol. Res. 102: 715. 1998.
Ascomata perithecial, non-stromatic, semi-immersed, gradually erumpent to almost superficial, scattered or clustered in small groups of 2–3, upright, obliquely oriented or lying horizontally on the host; venter 210–260 μm diam, 220–250(–300) μm high, black, subglobose with a flattened base and a broadly conical apex, often laterally flattened, flask-shaped when lying horizontally, with a papilla or short neck, broadly conical or cylindrical, apically truncate, central, eccentric or lateral, oriented upwards when ascomata lie horizontally. Ostiole periphysate. Ascomatal wall fragile, 9–15 μm thick, thicker at the apex up to 20 μm, 2-layered; outer layer consisting of dark brown, polyhedral, flattened cells of textura prismatica with opaque walls and sparse pores, outwards grading into small protruding cells, inner layer consisting of several rows of thin-walled, hyaline, flattened cells. Asci 125–150 × 11–14 μm (mean ± SD = 137 ± 9.4 × 12.6 ± 1.2 μm), cylindrical, short-stipitate, broadly rounded apically to obtuse, with a non-amyloid, discoid apical annulus 4.5–5.5 μm wide, 1.0–2.0 μm high. Paraphyses sparse, partially disintegrating at maturity, septate, branching, anastomosing, 4.0–9.5 μm wide. Ascospores (17.5–)19.5–23.5(–24) × (6.3–)7.0–8.5 μm, (mean ± SD = 21.4 ± 1.4 × 7.7 ± 0.5 μm), ellipsoidal, equilateral, straight, versicolorous, middle cells olivaceous brown to brown, containing numerous small guttules, polar cells smaller, hyaline, smooth, unequally 3-septate, slightly constricted at the septa, without sheath or appendages, arranged obliquely uniseriately in the ascus. Colonies in vivo diffuse, visible only as single scattered macroconidia arising from short, hyaline conidiogenous cells on vegetative mycelium near ascomata. Conidia (30–)33–41 × 15–17.5 μm, ellipsoidal, broadly rounded at the apex, tapering basally, dark brown, opaque, basal cell subhyaline to pale brown, (3–)5–6-septate, septa obscured by a darker band.
Culture characteristics — Colonies slow growing, reaching c. 8–10 mm diam on PDA after 21 d at 25 °C. Aerial mycelium brown (oac639), pale brown (aoc661) in the centre of the colony and on inoculum block, velvety, reverse brown (oac733). Aerial hyphae smooth, thin-walled, sparsely branched, hyaline to subhyaline 1.5–2.0 μm, submerged hyphae sometimes pale brown 2.0–3.0 μm wide. Conidiophores reduced to a monoblastic conidiogenous cell. Conidiogenous cells 4.5–7.0 × 5.0–8.0 μm, usually with several subtending cells, integrated, hyaline to subhyaline with a single conidiogenous locus. Conidia 32–36(–39) × (14.5–)16–17.5(–18.5) μm (mean ± SD = 34.5 ± 2.2 × 17.0 ± 1.0 μm), ellipsoidal to obovoid, straight or slightly curved, smooth, dark brown, 3–5-septate, with darker bands obscuring the septa, non-constricted at the septa, basal cell subhyaline 3.0–4.5 μm wide tapering to 2.5–3.0 μm.
Specimens examined. FRANCE, Midi-Pyrénées, Ariège, Rimont, valley of the Peyrau brook, c. 400 m asl, 23 Feb. 2008, on submerged wood, J. Fournier J.F. 08011 (PRM 933849, culture CBS 126792); ibid., 22 May 2009, submerged wood of Alnus glutinosa, J. Fournier J.F. 09127 (PRM 933851, culture CBS 126576); ibid., 19 Apr. 2010, submerged wood of Fraxinus excelsior, J. Fournier J.F. 10014; ibid., Vernajoul, Vernajoul brook, Pont Fagé, c. 350 m asl, on unidentified submerged wood, 2 July 2007, J. Fournier J.F. 07123 (PRM 933850).
Notes — Savoryella limnetica was originally collected on decaying wood submerged in freshwater in Taiwan and assigned to the genus based on its 3-septate ascospores and flattened apical apparatus (Chang et al. 1998). This species was recently repeatedly collected on submerged deciduous wood in southern France. Two living cultures were successfully obtained from isolated ascospores from fresh material.
Savoryella and Ascotaiwania are closely related, morphologically similar genera and their delimitation is based primarily on ascospore septation, morphology of the apical apparatus of the ascus and width of the paraphyses (see Discussion). The transfer of S. limnetica to Ascotaiwania is supported by molecular data and culture characters. The majority of Ascotaiwania species have 5–7-septate ascospores and only few are characterised by ascospores with three septa, i.e. A. hughesii, A. palmicola and A. sawadae. Helicoön farinosum and its sexual morph described as A. hughesii (Fallah et al. 1999), is a member of the Pleurotheciales. Ascotaiwania palmicola differs from A. limnetica by terrestrial habitat and affiliation to palm wood, asci with a conspicuous apical apparatus 4 × 5 μm and slender ascospores, 17.5–20 × 5.0–6.5 μm with polar mucilaginous appendages (Hyde 1995). Ascotaiwania sawadae can be compared to A. limnetica by ascomatal morphology, but differs by asci with a less flattened apical apparatus and larger and inequilateral ascospores 25–30 × 7.5–10 μm (Sivichai et al. 1998).
When observed in Congo red, the asci of A. limnetica revealed a conspicuous flattened apical annulus that stains deep red (Fig. 9k).
Pleurotheciella uniseptata (Matsush.) Seifert, comb. nov. — MycoBank MB813238; Fig. 11
Fig. 11.

Pleurotheciella uniseptata. a, b. Conidiophores in vivo; c. conidia in vivo; d. conidiophores on CMA; e. colony in vivo (a–c. DAOM 673210, d. DAOMC 250294, 21 d at 25 °C); a: PC; b–d. DIC. — Scale bars: a–d = 10 μm; e = 100 μm.
Basionym. Dactylaria uniseptata Matsush., Microfungi of the Solomon Islands and Papua-New Guinea: 19. 1971.
Colonies in vivo effuse, visible as solitary to 4–5 caespitose dark brown conidiophores with dry, whitish to greyish conidia. Sexual morph not observed. Conidiophores mostly 100–150 μm tall, 4.5–5.0 μm wide at the base, tapering to 3.0–4.0 μm wide, macronematous, unbranched, straight or sinuous, dark brown at the base, with walls up to 1.0 μm thick near the base, thinner towards the apex, cylindrical, smooth-walled or slightly granular or roughened, usually with a terminal node of denticles, but rarely extending through the original node with a new extension of the conidiophore. Conidiogenous cell 15–32 μm long, 2.5–3.5 μm wide at the base, 2.0–3.0 μm wide below the fertile zone, integrated, terminal, cylindrical or tapering towards tip, pale brown to subhyaline near base, hyaline towards apex, smooth-walled or slightly granular, polyblastic, forming conidia sympodially on conspicuous denticles 1.0–2.0 μm long, about 0.5 μm wide, sometimes slightly broader at base, occluded, fertile zone at first just a few denticles, but can expand into a node-like zone that is cylindrical to ellipsoidal in outline, usually with compact clusters of 4–15 denticles but sometimes extended, rarely geniculate, up to 5.0–9.0 × 3.0 μm, wide, or be constricted down to 1.5 μm, up to 15 denticles seen. Conidia 12.5–16.5 × 2.0–4.0 μm (mean ± SD = 14.1 ± 0.9 × 2.9 ± 0.5 μm), fusoid or slightly clavate, straight, rounded at the apex, obtuse and tapering towards base, hyaline, 1-septate with an inconspicuous central septum, often with 1–2 large guttules in each cell, smooth-walled, remains of denticle sometimes attached to seceded conidium.
Culture characteristics — Colonies reaching 8–10 mm diam on CMA after 21 d at 25 °C. Aerial mycelium absent, colony and reverse inconspicuous to white. Submerged hyphae 1.5–2.0 μm wide, hyaline, smooth-walled. Sporulation appears first on the inoculum of the colony, and later is sparsely present on the older parts of the new growth. Conidiophores 50–85 × 3.5–4.0 μm wide, slightly swollen at base to about 4 μm, semi-macronematous, pale brown, subhyaline towards the apex, unbranched, smooth-walled. Conidiogenous cells and conidia similar to those produced in vivo.
Specimen examined. CANADA, Ontario, Arnprior, MacNamara Trail, on decaying wet wood, 12 Oct. 2011, K.A. Seifert & G. White K.A.S. 4459 (DAOM 673210, culture DAOMC 250294).
Notes — Pleurotheciella uniseptata is known only from its asexual morph. Its occurrence on water saturated decayed wood is consistent with the ecology of the other two species now classified in this genus. Its conidia are of a similar length and septation to those of P. rivularia, but narrower and more uniformly fusiform rather than the often obovoidal shape of the latter species. The conidia of P. centenaria are also fusoid, but longer than the other two species and consistently 3-septate. Our specimen from Canada fits the description and illustration of Dactylaria uniseptata by Matsushima (1971) well, considering that the protologue was based on a culture grown on banana leaf agar. We note that De Hoog (1985) failed to obtain the holotype of D. uniseptata and we did not attempt to obtain it here. We have resisted the temptation to epitypify a Japanese species with a Canadian specimen and culture. Lectotypification with the drawings from the protologue would be a precondition to epitypification if the holotype is truly unavailable.
This is the first species of Pleurotheciella for which the conidiophores have been observed on the natural substrate. The protologue of the genus suggested that the conidiophores were dactylaria-like, but in P. uniseptata the conidiophores are macronematous and much more similar to those of Pleurothecium species. However, the conidiophores of P. uniseptata produced in culture lack dark basal cells and are rather similar to those produced by P. rivularia and P. centenaria in vitro (Fig. 11d). It seems possible that the conidiophores or all Pleurotheciella species would be macronematous in vivo. Morphologically, there are few if any characters to distinguish between the asexual morphs of Pleurotheciella uniseptata and some species classified in the hyphomycete genus Pleurophragmium. The two genera are clearly phylogenetically distinct, with Pleurophragmium parvisporum, the type of that genus, classified in the Papulosaceae, Sordariomycetes by Réblová (2009). A great morphological diversity of species are classified in Pleurophragmium (see key in D’Souza & Bhat 2012) and it is unlikely to be phylogenetically homogeneous.
DISCUSSION
The CPS (Conioscyphales/Pleurotheciales/Savoryellales) clade
The combined three- and six-gene phylogenetic analyses of the newly described genera Melanotrigonum and Adelosphaeria with members of the Savoryellales and other taxa related to Conioscypha and Pleurothecium revealed a robust monophylum in the Hypocreomycetidae (Fig. 1). It contains three nested monophyletic clades significantly supported by BI and ML methods, namely i) the Savoryellales; ii) a clade containing five species of Conioscypha; and iii) another clade that comprises several genera centred around Pleurothecium. The two latter clades represent distinct taxonomic groups at the ordinal systematic level and are introduced as the Conioscyphales and Pleurotheciales above. A sister relationship was revealed between the CPS (Conioscyphales/Pleurotheciales/Savoryellales) clade and a monophyletic strongly supported lineage of uncertain systematic position containing Ascotaiwania persoonii, Bactrodesmiastrum and the new genus Plagiascoma.
Members of the CPS and Bactrodesmiastrum clades share a few morphological features such as the absence of stromatic tissue or clypeus, similar anatomies of the ascomatal walls, thin-walled unitunicate asci with a distinct, non-amyloid apical annulus, paraphyses and symmetrical, transversely septate ascospores. The known asexual morphs are dematiaceous hyphomycetes with holoblastic conidiogenesis. Although the morphology of sexual morphs is more or less uniform and rather nondescript within each order, the observed variability in extension of conidiogenous cells and conidial morphology is characteristic of each order. In the CPS clade, pleomorphism is commonly observed, i.e. the ability of fungi to reproduce sexually and asexually and form independent spore-stages in the life cycle. All known life-histories discussed here were established experimentally, i.e. Ascotaiwania (Ranghoo & Hyde 1998, Sivichai et al. 1998, this study), Canalisporium (Sri-indrasutdhi et al. 2010), Conioscypha (Réblová & Seifert 2004, Zelski et al. 2014), Helicoön farinosum (Fallah et al. 1999), Pleurothecium (Fernández et al. 1999), Pleurotheciella (Réblová et al. 2012), Sterigmatobotrys (Réblová & Seifert 2011) and the new genera described in this study.
At the base of the monophyletic clade with the nested CPS and Bactrodesmiastrum clades, Flammispora bioteca is positioned on a separate branch (Fig. 1, 2). This species was collected on submerged leaves of the peat swamp palm Licuala longecalycata and is characterised by non-stromatic, black, immersed ascomata, clavate deliquescent asci without an apical annulus, subcylindrical to elongate-fusiform ascospores with a polar appendage and absence of paraphyses (Pinruan et al. 2004). Its asexual morph is unknown.
The Bactrodesmiastrum clade
Bactrodesmiastrum, based on B. obscurum, was described by Holubová-Jechová (1984) for dematiaceous hyphomycetes characterised by schizolytic conidial secession and a formation of conidiogenous cells related to the maturation of brown, septate conidia. When the conidium matures at the tip of the conidiogenous cell, a new monoblastic conidiogenous cell is borne near the previous one on repent basal hyphae, followed by formation of other conidiogenous cells in the same manner. No DNA sequences are available of the type species of Bactrodesmiastrum. The sexual state of Bactrodesmiastrum is unknown (Holubová-Jechová 1984, Hernández-Restrepo et al. 2013, 2015) and no conidia or conidiogenous cells were observed on the type and other herbarium material of its closest ascoma-producing sibling A. persoonii (Fallah et al. 1999). We prefer to avoid proposing a new genus for A. persoonii or its new combination in Bactrodesmiastrum, based on current DNA sequence data, until similarities in the life histories of these two taxa are proven or disproven experimentally.
Conioscyphales
The Conioscyphales comprises a single genus Conioscypha with 12 species from freshwater and terrestrial habitats. Conioscyphascus based on C. varius was originally proposed for fungi with Conioscypha asexual morphs by Réblová & Seifert (2004). Conioscypha exhibits a unique mode of conidiogenesis with multiple, conspicuous collarettes forming a multilamellar structure around the blastic conidiogenous locus of the intercalary conidiogenous cells (Shearer & Motta 1973). It is characterised by inconspicuous perithecial ascomata that are typically immersed to semi-immersed, hyaline, subhyaline to pale orange with a papilla or long upright neck, coriaceous, waxy ascomatal wall, cylindrical-clavate stipitate asci with a pronounced non-amyloid apical annulus, filiform paraphyses and fusiform to fusiform-navicular, septate, hyaline ascospores.
Nine species are known as apparently asexual (Von Höhnel 1904, Shearer 1973, Matsushima 1975, 1993, 1996, Kirk 1984, Udagawa & Toyazaki 1983, Chen & Tzean 2000, Crous et al. 2014) and only two have experimentally proven link between the sexual and asexual morphs, i.e. C. varia (Réblová & Seifert 2004) and C. peruviana (Zelski et al. 2014). A third sexually reproducing species, Conioscyphascus gracilis, was recently transferred to Conioscypha (Zelski et al. 2014).
Pleurotheciales
Six monophyletic clades that include species of eleven genera were nested in the clade that we describe above as the Pleurotheciales (Fig. 2). Members of the Pleurotheciales share dark, papillate, glabrous or sparsely setose perithecia, upright or lying horizontally to the host, asci with a distinct non-amyloid apical annulus, filiform paraphyses that disintegrate partially at maturity and fusiform to ellipsoidal, septate, hyaline ascospores. Only ascospores of the sexual morph of Helicoön farinosum are versicolorous with brown middle cells and hyaline polar cells.
The variation in the details of holoblastic conidiogenesis correlates with clades recovered within the order. Rhexolytic conidial secession either on short denticles or rachis on sympodially proliferating conidiogenous cells occurs in Helicoön farinosum, Phaeoisaria, Melanotrigonum, Pleurothecium, Pleurotheciella and Sterigmatobotrys. This type of conidiogenesis is characteristic of Clades I, IV, V, VI and partially occurs in Clades II and III. Schizolytic conidial secession on a single locus on percurrently regenerating conidiogenous cells is characteristic of Brachysporiella sensu Ellis (Ellis 1959). The same type of secession but on monoblastic or solitary thallic conidiogenous cells is typical of Phragmocephala. Both latter genera are positioned in Clade II. In Taeniolella, a sister of Sterigmatobotrys in Clade III, the dark brown macroconidia are formed on monoblastic conidiogenous cells in dry, acropetal chains, while the apex of the conidium may develop into a fertile penicillate head with sympodially elongating conidiogenous cells similar to Sterigmatobotrys (see further under Taeniolella).
The nondescript morphology of sexual characters of members of the Pleurotheciales makes their correct placement in the Sordariomycetes difficult and significantly hinders their identification and even distinction from each other. Without cultivation and/or molecular data their correct systematic placement is challenging. The presence of conspicuous asexual morphs in intimate juxtaposition to ascomata on the natural substratum helps identification of several genera only. Some species of Pleurotheciella do not form conidiophores in vivo, only reduced, hyaline to subhyaline conidiophores in the axenic culture. Genera like Adelosphaeria and Plagiascoma, the latter is positioned in the Bactrodesmiastrum clade outside the Pleurotheciales, do not even form typical asexual morphs. They produce brown, ellipsoidal to globose, non-septate cells arising blastically from vegetative hyphae or other cells in the axenic culture.
Members of Chaetosphaeria (Chaetosphaeriales) are morphologically similar to Pleurothecium, Pleurotheciella and Sterigmatobotrys of the Pleurotheciales, especially species with Menispora asexual morphs, e.g. C. ciliata, C. ovoidea, C. pulviscula or C. tortuosa (Holubová-Jechová 1973, Réblová et al. 2006, Réblová & Seifert 2008). They possess brown, upright, papillate ascomata, fusiform, 3-septate, hyaline ascospores in cylindrical-clavate asci with distinct apical annulus and their phialidic asexual morphs are often absent on the host. Several freshwater genera such as Aquaticola, Annulatascus and Annulusmagnus (Ho et al. 1999, Hyde 1992a, Campbell & Shearer 2004) can be compared with Adelosphaeria, Melanotrigonum, Pleurothecium, Pleurotheciella and Sterigmatobotrys based on morphology of ascomata, asci, ascospores and paraphyses. Species of Aquaticola have miniature, coriaceous ascomata lying horizontally to the host, asci with inconspicuous non-amyloid apical annulus and septate or non-septate, hyaline ascospores (Ho et al. 1999, Tsui et al. 2003). Annulatascus and Annulusmagnus are easily distinguished by asci with a conspicuous, non-amyloid apical annulus and relatively large, septate, fusiform ascospores with a sheath or appendages in the former taxon, arranged 1-seriately or obliquely 1-seriately in the ascus. Their asexual morphs are unknown and when isolated from ascospores, sterile mycelium, or in the case of Annulusmagnus triseptatus abundant fertile ascomata (M. Réblová, pers. obs.) are formed in vitro. Phomatospora, whose taxonomic placement in the Sordariomycetidae is uncertain (Lumbsch & Huhndorf 2010), is another perithecial ascomycete that can be compared with genera of the Pleurotheciales. Its species are distinguishable by occurrence primarily on submerged herbaceous stems, rarely on wood in freshwater and marine habitats, immersed ascomata with thickened wall surrounding the ostiolum and hyaline, longitudinally striate non-septate ascospores enclosed in mucilaginous sheath or with bipolar appendages (e.g. Hyde 1988, 1992b, Fallah & Shearer 1998, Fournier & Lechat 2010). Only Phomatospora berkeleyi, the type species, and P. arenaria produce sporothrix-like asexual morphs with holoblastic denticulate conidiogenesis in axenic culture (Rappaz 1992).
Savoryellales
The Savoryellales was placed in the Hypocreomycetidae based on DNA sequences of six ribosomal and protein-coding loci (Boonyuen et al. 2011). It forms a well-supported lineage that includes saprobic, lignicolous species from terrestrial, marine, brackish and freshwater environments and water-cooling towers (e.g. Jones & Eaton 1969, Minoura & Muroi 1978, Hyde & Jones 1988, Chang et al. 1998, Ranghoo & Hyde 1998). Although Ranghoo (1998) introduced the family Savoryellaceae as a member of the Halosphaeriales in her PhD Thesis, a valid description was never published. The family was formally introduced recently as Savoryellaceae (Jaklitsch & Réblová 2015).
As now delimited, the Savoryellales comprises three genera, Ascotaiwania, Canalisporium and Savoryella. Ascotaiwania is polyphyletic in our analyses, although the genus appeared monophyletic in three previous studies (Campbell & Shearer 2004, Hernández-Restrepo et al. 2013, 2015). The latter results were inadvertently distorted by the inclusion of species of Ascotaiwania that only represent the CPS and Bactrodesmiastrum clades on a small scale. In our multilocus phylogenies (Fig. 1, 2) the core of Ascotaiwania in the Savoryellales is centred around the type species A. lignicola (Sivanesan & Chang 1992) and three other species. Helicoön farinosum (as A. hughesii, Fallah et al. 1999) is nested in the Pleurotheciales, while A. persoonii (Fallah et al. 1999) is in a strongly supported monophyletic clade with Bactrodesmiastrum and Plagiascoma basal to the CPS clade.
Genera of the Savoryellales share a similar morphology of dark, minute perithecial ascomata with elongated, dark or subhyaline neck, often oblique or lying horizontally on the host with the neck facing upwards, asci with a non-amyloid apical annulus, partly deliquescing paraphyses and ellipsoidal to fusiform, transversely septate, versicolorous ascospores. The generic delimitation of Ascotaiwania and Savoryella is narrow and for two decades was based predominantly on ascospore septation, and the morphologies of paraphyses and the ascal apex, i.e. size and shape of the apical annulus and presence or absence of apical thickening. The ascal apex of Savoryella was variously interpreted in different studies, by authors studying different species. In the protologue of the type species S. lignicola, the ascal apex was described as apically thickened with a pore (Jones & Eaton 1969). Sivanesan & Chang (1992) separated Ascotaiwania from Savoryella by an unthickened ascal apex with a distinct apical annulus and ascospores with more than three septa, while delimiting Savoryella for species lacking an apical ring and having 3-septate ascospores. Later, several other species were introduced to the genus, e.g. S. aquatica (Hyde 1993) and S. limnetica (Chang et al. 1998), characterised by a thickened ascal apex containing apical annulus with a pore. Read et al. (1993) based their distinction of Ascotaiwania and Savoryella on ultrastructural observations and used the term ‘apical apparatus’ to describe the complex structure of the ascal apex of these fungi. They characterised species of Ascotaiwania by ascal apical apparatus comprising an annulus with a protrusion (pendant) and plugged pore, whereas in species of Savoryella the ascus apex was described as thickened with a pore, but lacking a pendant-like protrusion. Chang et al. (1998) also used characters of paraphyses to delimit the genera, i.e. narrow, filiform, early deliquescing filaments up to 2 μm wide in Ascotaiwania vs filaments consisting of broad, partially disintegrating cells up to 8 μm wide in Savoryella. Sri-indrasutdhi et al. (2010) introduced another morphologically similar genus, Ascothailandia, as the sexual state of Canalisporium and distinguished it from Savoryella by its conspicuous apical annulus. Recently, Boonyuen et al. (2011) modified the generic concept of Savoryella and accepted species with 3-septate ascospores and comparatively flattened apical ring.
The transfer of S. limnetica to Ascotaiwania proposed above is based on molecular evidence and an experimentally proven life history. The micromorphological characters of S. limnetica, i.e. flattened apical annulus, cylindrical, septate, disintegrating paraphyses 4.0–9.5 μm wide and 3-septate ascospores, do not fit well with the long-held morphology-based concepts of either genus. Stable delimitation of Ascotaiwania and Savoryella will require re-evaluation of all sexual and asexual morphological characters and concentrated sampling filtered through the optics of multigene phylogenetics.
Asexual morphs associated with the Savoryellales were described for Canalisporium grenadoideum (as Ascothailandia grenadoidea sexual morph, Sri-indrasutdhi et al. 2010) and three species of Ascotaiwania were linked with Brachysporiella-like dematiaceous hyphomycetes, A. mitriformis and A. sawadae (as Monotosporella, Ranghoo & Hyde 1998, Sivichai et al. 1998) and A. limnetica (this study). With some reservations Acarocybiopsis was suggested as another suitable genus for asexual morphs of Ascotaiwania (Réblová & Seifert 2004). They are characterised by semi-macronematous conidiophores often reduced to conidiogenous cells with a single locus and brown macroconidia. Conidia are either cheiroid, dictyoseptate with pores between cells and conidiogenous cells arise from sporodochia in Canalisporium. The asexual morphs of Ascotaiwania produce aleuroconidium-like, transversely septate macroconidia with darker bands around septa and a few rhizoids arising from subtending cells beneath the monoblastic conidiogenous cell.
In our analysis, the dematiaceous hyphomycete Triadelphia uniseptata nested within the monophyletic Ascotaiwania clade as a sister to A. mitriformis. Triadelphia, based on T. heterospora, was introduced for fungi from freshwater and brackish environments and characterised by conidiophores reduced to subglobose, subhyaline to dematiaceous conidiogenous cells, schizolytic conidial secession and conidia produced blastically from a single locus (Shearer & Crane 1971). Conidia of species of Triadelphia are brown or versicolorous often with one or two polar cells paler than the middle ones, septate, usually with darker bands obscuring several septa. Although T. heterospora was described with two morphologically distinct types of conidia, currently eight types are known (Constantinescu & Samson 1982), but these other asexual morphs have never been formally named. The gregarious to caespitose, globose to subglobose to ampulliform conidiogenous cells borne directly on vegetative hyphae are the hallmark of Triadelphia. They are also remarkably similar to cylindrical to lageniform aggregated conidiogenous cells of Bactrodesmiastrum. The morphology of larger, broad to ellipsoidal, brown, septate conidia of T. heterospora and their conidiogenesis illustrated in the protologue (Shearer & Crane 1971: f. 9c, f, g) resembles conidia and conidiogenesis of A. limnetica (Fig. 10c–h) that we observed in axenic culture on PDA. The ampulliform conidiogenous cells are absent and conidia arise directly from mycelium or a small monoblastic conidiogenous cell with several supporting cells. Triadelphia comprises 17 species, but phylogenetic placement of its type species is unknown. The only available ITS and nuc28S rDNA sequences in the GenBank belong to T. pulvinata and they show affinity with members of the Microascales (Edathodu et al. 2013). The position of T. uniseptata in the Savoryellales shown here demonstrates that the present concept of Triadelphia is polyphyletic, and that the application of this generic name, and the redisposition of its species, requires much improved sampling.
The ascoma centrum in the Hypocreomycetidae
In members of the Hypocreomycetidae, the centrum consists of several types of interthecial filaments. The other two subclasses of the Sordariomycetes, Sordariomycetidae and Xylariomycetidae include either only paraphyses and periphyses in the ostiolum or paraphyses are lacking in some groups. Apical, lateral and centripetal paraphyses occur in members of the Hypocreales (e.g. Samuels 1973, Mhasker & Rao 1976, Jaklitsch 2009, Jaklitsch & Voglmayr 2014). Filaments consisting of wide, inflated, early disintegrating cells interspersed among the asci occur in the Bertiaceae and Chaetosphaerellaceae of the Coronophorales (Réblová 1999, Huhndorf et al. 2004). A hamathecium consisting of catenophyses, i.e. pseudoparenchymatous cells that break up to form chains of large, thin-walled, early dissolving cells interspersed among asci or the pseudoparenchyma may completely disappear in mature ascomata, is typical of members of the Halosphaeriaceae of the Microascales (Spatafora et al. 1998, Sakayaroj et al. 2011). A pseudoparenchymatous centrum occurs in the Melanosporales (Goh & Hanlin 1994, Samuels & Blackwell 2001). A reticulate network of filiform, branching and anastomosing filaments attached at the top and bottom of the cavity uniquely characterises the Reticulascaceae of the Glomerellales while in members of other two families, Australiascaceae and Glomerellaceae, sparse septate filaments occur (Samuels & Müller 1978, Sivanesan & Alcorn 2002, Réblová et al. 2011). Numerous unbranched filaments attached to the top and bottom of the ascomatal cavity occur in members of the Torpedosporales except for Marinokulati chaetosa, where the filaments are apically free (Jones et al. 2014, 2015). In some groups, a hamathecium is lacking, e.g. in the Scortechiniaceae and Nitschkia of the Coronophorales (Huhndorf et al. 2004) or in some members with cleistothecial ascomata of the Microascales. The presence of periphyses in genera of the Coronophorales is variable and depends on how the apex of ascomata is formed, whether it contains a Quellkorper (Nannfeldt 1975) and whether it is ostiolate or non-ostiolate (Huhndorf et al. 2004).
Members of the CPS clade represent the only three orders in the Hypocreomycetidae defined by the presence of apically free paraphyses in the ascomatal centrum. These sterile, filiform, septate filaments emerge from the subhymenium either interspersed among the asci, e.g. in Ascotaiwania, Conioscypha, Melanotrigonum, Pleurotheciella, Savoryella and Sterigmatobotrys, or form separate tuft-like structures, e.g. Pleurothecium. Paraphyses are usually longer than the asci and may disintegrate at maturity; for example in some species of Savoryella or Ascotaiwania they disintegrate rapidly and are difficult to observe.
Recently, the new order Pisorisporiales was introduced for predominantly aquatic fungi, which morphologically mimic members of the Annulatascaceae in ascoma and ascospore characters, and the Amphisphaeriaceae in a conspicuous, amyloid apical annulus and non-stromatic ascomata (Réblová et al. 2015). The order is isolated on a separate branch as a sister to the Hypocreomycetidae but without statistical support. The Pisorisporiales represents another group related to this subclass and characterised by filiform, septate, partly disintegrating paraphyses interspersed among asci, but densely branching and anastomosing above their apices in the ascoma cavity. Although the two species of Pisorisporium, P. cymbiforme and P. glaucum, were described from wood submerged in fresh-water, several recent collections of P. cymbiforme were made in terrestrial habitats in the Czech Republic, suggesting that the fungus might be widespread. The new nuc18S rDNA and rpb2 sequences of terrestrial strains are listed in Table 1.
Pleurotheciales: The polyphyletic genera Helicoön, Phaeoisaria, Pleurothecium and Taeniolella
Helicoön
Several genera now classified in the Pleurotheciales appear polyphyletic based on molecular phylogenies. Helicoön farinosum, which has hyaline, coiled, septate conidia formed holoblastically on short denticles, is the only representative with helicosporous conidia in the Pleurotheciales and in the whole CPS clade. It was experimentally linked with its sexual state Ascotaiwania hughesii (Fallah et al. 1999) and in our phylogeny it is nested in Clade I as a sister to Brachysporiella setosa. We confirmed the phylogenetic position of H. farinosum (DAOM 241947) with collections, cultures and sequences made in Canada (Réblová et al. 2012). Although the correct species epithet for this holomorphic fungus would be ‘farinosum’, whether the generic assignment should be Helicoön is unclear pending confirmation of the phylogenetic placement and classification of the type species H. sessile. The genus Helicoön sensu Goos et al. (1986) was shown to be polyphyletic with DNA sequences of two nuc rDNA loci by Tsui & Berbee (2006), but H. sessile was not included. The only available ITS rDNA sequence of this species (U72605, Pfister et al. 1997) shows 99 % similarity with the ITS sequence of Sarocladium kiliense of the Hypocreales (KP132606, Irinyi et al. 2015), an unlikely relationship suggestive of a mislabelled or contaminated culture. Other species of Helicoön were placed in the Pleosporales, Tubeufiales and Venturiales of the Dothideomycetes (Tsui & Berbee 2006).
Phaeoisaria
Phaeoisaria is a dematiaceous hyphomycete genus with species producing indeterminate synnemata with septate or non-septate ellipsoidal, obovoidal, fusiform-cylindrical or falcate conidia formed on a sympodially extending rachis, occurring on decaying wood, plant debris or soil sediments (e.g. Sutton 1973, Deighton 1974, Castañeda et al. 2002, Seifert et al. 2011, Mel’nik 2012, Cheng et al. 2014, Crous et al. 2015). The genus was proposed by Von Höhnel (1909) with the only species Ph. bambusae. It was originally described as an asexual state of Neopeckia bambusae, inferred from the intimate juxtaposition of synnemata and ascomata. Based on his revision of type and herbarium material, Deighton (1974) considered Ph. bambusae a synonym of Ph. clematidis. He compiled an extensive synonymy of the latter species, distinguishing it from morphologically similar Ph. magnifica, which has broader conidia. Deighton’s concept of Ph. clematidis seems to represent a complex of several phylogenetic species.
Phaeoisaria now includes 19 species, five of which were analysed in our study. The sampled species form a strongly supported monophyletic clade in the Pleurotheciales that includes species with synnemata and conidiophores formed in fascicles. In our analysis, P. clematidis is represented by two strains isolated from bark and senescent flower heads of Protea.
Phaeoisaria curvata is the only described mononematous species; it was isolated from leaves of Parinari capense and its wild type is unknown (De Hoog & Papendorf 1976). The nuc28S sequence of the ex-type strain CBS 153.72 (sequence in the CBS strain database) shows affinity with taxa of the Sordariomycetidae.
Although the majority of Phaeoisaria species are asexual, including all species in our analyses, several perithecial ascomycetes have been linked with Phaeoisaria-like asexual states. In the Sordariomycetes, Lentomitella and Rhamphoria produce sparsely branched, mononematous conidiophores with aseptate conidia borne on a short rachis in culture (Müller & Samuels 1982, Réblová 2006). Two genera of the Diatrypaceae, Eutypella (as Peroneutypella, Deighton 1974) and Pareutypella (Ju & Rogers 1995), were linked with Phaeoisaria-like synnematous asexual states. For these connections, the morphologically similar synnematous genus Harpographium, typified by the asexual state of Eutypella scoparia, should be considered.
Although Phaeoisaria is usually considered non-pathogenic to human beings, two cases of inflammation of the eye’s cornea called keratitis were attributed to Phaeoisaria sp. (Chew et al. 2010) and Ph. clematidis (Guarro et al. 2000). The former pathogenic strain Phaeoisaria sp. was included in our analysis and is a sister taxon to two saprobic strains of Ph. clematidis with strong branch support.
Pleurothecium
Pleurothecium includes fungi with dematiaceous, macronematous, unbranched conidiophores and holoblastic, hyaline to subhyaline, sympodially extending conidiogenous cells with a conspicuous rachis of denticles and hyaline, septate conidia. The sexual morph is known only for the very common P. recurvatum, the type species (as Carpoligna pleurothecii, Fernández et al. 1999). Of the eight species assigned to the genus, only three have been studied with DNA sequence data. Pleurothecium recurvatum and P. semifecundum represent the core of the genus and form a strongly supported monophyletic clade in the Pleurotheciales, while P. obovoideum is nested within another clade and sister to Brachysporiella setosa. The asexual morph of P. semifecundum lacks macronematous conidiophores in culture and sporulates sparsely; whether its wild type would better match the distinctive conidiogenous apparatus of P. recurvatum remains unknown.
Pleurothecium obovoideum, originally described in Ramichloridium, is known only from culture and it was isolated from a dead leaf of Pasania edulis (Matsushima 1975). It is characterised by reduced, septate conidiophores, sympodially proliferating conidiogenous cells with a short rachis giving rise to 2–3 denticles and ellipsoidal to obovate, pale brown, non-septate conidia formed singly or in short chains. The morphology is rather nondescript and we prefer to avoid introducing a new genus for this species, until either the wild type is collected or relationship with other morphologically similar taxa is revealed. Based on its morphology, P. obovoideum is similar to Rhinocladiella mackenziei (Chaetothyriales), a pathogen causing severe cerebral phaeohyphomycosis in humans (Sutton et al. 1998). It also resembles members of Subramaniomyces (Xylariales, Crous et al. 2007) and Pterygosporopsis (Kirk 1983), whose phylogenetic placement is unknown.
Taeniolella
Taeniolella exilis, the type of the genus, is commonly found on decaying wood and bark of Betula (Hughes 1958, Ellis 1971). During a revision of the type material of T. exilis by Jones et al. (2002), a penicillately branched conidiophore was observed as an extension of the terminal macroconidia. A similar penicillate conidiophore was observed in two other species, T. longissima and T. rudis (Hughes 1980, Jones et al. 2002). The latter taxon was shown to be closely related to Sterigmatobotrys macrocarpa of the Pleurotheciales, whose asexual state is characterised by similar penicillate conidiophores with several series of branches and metulae terminating macronematous conidiophores (Réblová & Seifert 2011). However, brown, septate Taeniolella macroconidia were not observed in axenic cultures obtained from conidia or ascospores of S. macrocarpa.
Several other species of Taeniolella are positioned in distantly related groups. Taeniolella-like conidia were obtained in a culture derived from ascospores of the freshwater ascomycete Chaetorostrum quincemilensis, tentatively placed in the Annulatascaceae (Zelski et al. 2011). Shearer et al. (2009) showed the strain of T. alta (CBS 488.80) nested in a clade with Diaporthe angelicae and Phomopsis sp., and the ex-type strain of T. typhoides (CCM F-10198) in the Lingdomycetaceae of the Pleosporales. A taeniolella-like fungus was isolated from the rhizosphere soil of strawberry, producing a phialophora-like asexual state on vegetative hyphae or directly on macroconidia in vitro, and described as T. phialosperma (Watanabe 1989, 1992). The ITS sequences of two non ex-type strains of T. phialosperma deposited in GenBank (KF703925, GU966521, unpubl.) indicate a relationship with members of the Sordariales. Finally, the sexual morph of a Taeniolella sp. with ascolocular ascoma development was classified as Mytilinidion gemmigenum (Mytilinidiales, Minter & Holubová-Jechová 1981). These inconsistencies suggest that the generic concept of Taeniolella requires increased taxon sampling and investigation with molecular methods.
A case of human subcutaneous phaeohyphomycosis caused by T. exilis species was reported by Alonso et al. (1993). While the pathogenic strain of T. exilis isolated from a human skin lesion (strain IP 2199.93) was shown closely related to Ochrocladosporium elatum (CBS 146.33) of the Pleosporales by Masclaux et al. (1995), the placement of the wood-inhabiting strain of T. exilis resembling T. rudis (Pleurotheciales) has yet to be confirmed with molecular sequence data.
Acknowledgments
This study was supported by the Project of the National Foundation of the Czech Republic (GA 506/12/0038), and as a long-term research development project of the Institute of Botany, Academy of Sciences No. RVO 67985939, and of the Institute of Microbiology, Academy of Sciences No. RVO 61388971. We thank Walter Gams for helpful suggestions on names of the new taxa proposed in our study.
Footnotes
In keeping with the tenets of the new International Code on the Nomenclature of algae, fungi and plants, we hereafter routinely use the oldest generic name for holomorphs; in some cases, this was originally the name of an asexual morph.
REFERENCES
- Abdel-Wahab MA, Abdel-Aziz FA, Mohamed SS, et al. 2011. Annulatascus nilensis sp. nov., a new freshwater ascomycete from the river Nile, Egypt. IMA Fungus 2: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso i Tarrés C, Heures JK, Gudho E, et al. 1993. Subcutaneous phaeohyphomycosis caused by Taeniolella exilis. 1st. Congress CEMM, Paris, no. 124 (Abstract). [Google Scholar]
- Boonyuen N, Chuaseeharonnachai C, Suetrong S, et al. 2011. Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella. Mycologia 103: 1351–1371. [DOI] [PubMed] [Google Scholar]
- Campbell J, Shearer CA. 2004. Annulusmagnus and Ascitendus, two new genera in the Annulatascaceae. Mycologia 96: 822–833. [DOI] [PubMed] [Google Scholar]
- Castañeda Ruíz RF, Velazquez S, Cano J, et al. 2002. Phaeoisaria aguilerae anam. sp. nov. from submerged wood in Cuba with notes and reflections on the genus Phaeoisaria. Cryptogamie, Mycologie 23: 9–18. [Google Scholar]
- Chang HS, Hsieh YS, Jones EBG, et al. 1998. Aquatic ascomycota: new freshwater species of Ascotaiwania and Savoryella from Taiwan. Mycological Research 102: 709–718. [Google Scholar]
- Chen JL, Tzean SS. 2000. Conioscypha taiwaniana sp. nov. and several new records of the genus from Taiwan. Botanical Bulletin of the Academia Sinica (Taipei) 41: 315–322. [Google Scholar]
- Cheng XL, Wei L, Zhang TY. 2014. A new species of Phaeoisaria from intertidal marine sediment collected in Weihai, China. Mycotaxon 127: 17–24. [Google Scholar]
- Chew HF, Jungkind DL, Mah DY, et al. 2010. Post-traumatic fungal keratitis caused by Carpoligna sp. Cornea 29: 449–452. [DOI] [PubMed] [Google Scholar]
- Constantinescu O, Samson R. 1982. Triadelphia, a pleomorphic genus of hyphomycetes. Mycotaxon 15: 472–486. [Google Scholar]
- Crous PW, Braun U, Schubert K, et al. 2007. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2015. Fungal Systematics and Evolution: FUSE 1. Sydowia 67: 81–118. [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer ZW, Seifert KA, Wingfield MJ. 2013. The ophiostomatoid fungi: their dual position in the Sordariomycetes. In: Ophiostomatoid fungi: expanding frontiers. CBS Biodiversity Series 12: 1–19. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands. [Google Scholar]
- De Hoog GS. 1985. Taxonomy of the Dactylaria complex IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Studies in Mycology 26: 1–60. [Google Scholar]
- De Hoog GS, Papendorf MC. 1976. The genus Phaeoisaria. Persoonia 8: 407–414. [Google Scholar]
- Deighton FC. 1974. Four synnematous hyphomycetes. Transactions of British Mycological Society 62: 243–252. [Google Scholar]
- D’Souza MA, Bhat DJ. 2012. A new species of Pleurophragmium from India. Mycotaxon 119: 477–482. [Google Scholar]
- Edathodu J, Al-Abdely HM, Althawadi S, et al. 2013. Invasive fungal infection due to Triadelphia pulvinata in a patient with acute myeloid leukemia. Journal of Clinical Microbiology 51: 3426–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MB. 1959. Clasterosporium and some allied dematiaceae-phragmosporae. II. Mycological Papers 72: 1–75. [Google Scholar]
- Ellis MB. 1971. Dematiaceous hyphomycetes. CAB Commonwealth Mycological Institute, Kew, England. [Google Scholar]
- Fallah PM, Crane JL, Shearer CA. 1999. Freshwater ascomycetes: two new species of Ascotaiwania from North America. Canadian Journal of Botany 77: 87–92. [Google Scholar]
- Fallah PM, Shearer CA. 1998. Freshwater ascomycetes: Phomatospora spp. from lakes in Wisconsin. Mycologia 90: 323–329. [Google Scholar]
- Fernández FA, Lutzoni FM, Huhndorf SM. 1999. Teleomorph-anamorph connections: the new pyrenomycetous genus Carpoligna and its Pleurothecium anamorph. Mycologia 91: 251–262. [Google Scholar]
- Fournier J, Lechat C. 2010. Phomatospora luteotingens sp. nov., a new aquatic species of Phomatospora from France and Spain. Mycosphere 1: 39–43. [Google Scholar]
- Gams W, Hoekstra ES, Aptroot A. 1998. CBS course of mycology, 4th edn Baarn, The Netherlands: Centraalbureau voor Schimmelcultures. [Google Scholar]
- Geiser DM, Frisvad JC, Taylor JW. 1998. Evolutionary relationships in Aspergillus section Fumigati inferred from partial β-tubulin and hydrophobin DNA sequences. Mycologia 90: 831–845. [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh TK, Hanlin RT. 1994. Ascomatal development in Melanospora zamiae. Mycologia 86: 357–370. [Google Scholar]
- Goos RD, Abdullah SK, Fisher PJ, et al. 1986. The anamorph genus Helicoön. Transactions of British Mycological Society 87: 115–122. [Google Scholar]
- Guarro J, Vieira LA, De Freitas D, et al. 2000. Phaeoisaria clematidis as a cause of Keratomycosis. Journal of Clinical Microbiology 38: 2434–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell RR. 1993. Collection of small subunit (16 S and 16 S-like) ribosomal RNA structures. Nucleic Acids Research 21: 3051–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell RR, Gray MW, Schnare MN. 1993. A compilation of large subunit (23 S and 23 S-like) ribosomal RNA structures. Nucleic Acids Research 21: 3055–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. 1999. BioEdit 5.0.9: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, et al. 2015. Emendation of the genus Bactrodesmiastrum (Sordariomycetes) and description of Bactrodesmiastrum monilioides sp. nov. from plant debris in Spain. Mycological Progress: doi 10.1007/s11557-015-1067-6. [Google Scholar]
- Hernández-Restrepo M, Mena-Portales J, Gené J, et al. 2013. New Bactrodesmiastrum and Bactrodesmium from decaying wood in Spain. Mycologia 105: 172–180. [DOI] [PubMed] [Google Scholar]
- Ho WWH, Tsui CKM, Hodgkiss IJ, et al. 1999. Aquaticola, a new genus of Annulatascaceae from freshwater habitats. Fungal Diversity 3: 87–97. [Google Scholar]
- Holubová-Jechová V. 1973. Lignicolous hyphomycetes from Czechoslovakia. 4. Menispora. Folia Geobotanica et Phytotaxonomica 8: 317–336. [Google Scholar]
- Holubová-Jechová V. 1984. Bactrodesmiastrum, a new genus of lignicolous hyphomycetes. Folia Geobotanica et Phytotaxonomica 19: 103–106. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hughes SJ. 1958. Revisiones Hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 727–836. [Google Scholar]
- Hughes SJ. 1980. Taeniolella rudis. Fungi Canadenses No. 185. National Mycological Herbarium, Agriculture Canada, Ottawa. [Google Scholar]
- Huhndorf SM, Miller AN, Fernandez FA. 2004. Molecular systematics of the Coronophorales and new species of Bertia, Lasiobertia and Nitschkia. Mycological Research 108: 1384–1398. [DOI] [PubMed] [Google Scholar]
- Hyde KD. 1988. Phomatospora acrostichii sp. nov., a marine fungus on pinnae of Acrostichum speciosum. Transactions of the British Mycological Society 90: 135–138. [Google Scholar]
- Hyde KD. 1992a. Tropical Australian freshwater fungi I. Annulatascus velatispora gen. et sp. nov., A. bipolaris sp. nov. and Nais aquaticola sp. nov. (Ascomycetes). Australian Systematic Botany 5: 117–124. [Google Scholar]
- Hyde KD. 1992b. Intertidal fungi from Candelia candel including Phomatospora kandelae sp. nov. Transactions of the Mycological Society of Japan 33: 313–316. [Google Scholar]
- Hyde KD. 1993. Tropical Australian freshwater fungi. V. Bombardia sp., Jahnula australiensis sp. nov., Savoryella aquatica sp. nov. and S. lignicola sp. nov. Australian Systematic Botany 6: 161–167. [Google Scholar]
- Hyde KD. 1995. Fungi from palms. XXII. A new species of Ascotaiwania. Sydowia 47: 213–216. [Google Scholar]
- Hyde KD, Jones EBG. 1988. Marine mangrove fungi. P.S.Z.N.I. Marine Ecology 9: 15–33. [Google Scholar]
- Irinyi L, Serena C, Garcia-Hermoso D, et al. 2015. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database – the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Medical Mycology 53: 313–337. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. 2009. European species of Hypocrea. Part I. The green-spored species. Studies in Mycology 63: 1–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Réblová M. 2015. Savoryellaceae Jaklitsch & Réblová. Index Fungorum 209: 1. [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2014. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia 33: 182–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EBG, Eaton RA. 1969. Savoryella lignicola gen. et sp. nov. from water-cooling towers. Transactions of British Mycological Society 52: 161–174. [Google Scholar]
- Jones EBG, Eaton RA, Somrithipol S. 2002. Taeniolella rudis and Taeniolella longissima sp. nov. with secondary sympodioconidia from freshwater habitats. Mycoscience 43: 201–206. [Google Scholar]
- Jones EBG, Suetrong S, Cheng W-H, et al. 2014. An additional fungal lineage in the Hypocreomycetidae (Falcocladium species) and the taxonomic re-evaluation of Chaetosphaeria chaetosa and Swampomyces species, based on morphology, ecology and phylogeny. Cryptogamie, Mycologie 35: 119–138. [Google Scholar]
- Jones EBG, Suetrong S, Sakayaroj J, et al. 2015. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Diversity 73: 1–72. [Google Scholar]
- Ju YM, Rogers JD. 1995. Pareutypella gen. nov. for two long-ostiolate pyrenomycetes from Taiwan. Mycologia 87: 891–895. [Google Scholar]
- Kirk PM. 1983. New or interesting microfungi X. Hyphomycetes on Laurus nobilis leaf litter. Mycotaxon 18: 259–298. [Google Scholar]
- Kirk PM. 1984. New or interesting microfungi XII. A new species of Conioscypha (Hyphomycetes). Transactions of British Mycological Society 82: 177–178. [Google Scholar]
- Larget B, Simon DL. 1999. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16: 750–759. [Google Scholar]
- Lee SB, Taylor JW. 1990. Isolation of DNA from fungal mycelium and single spores. In: Innis MA, Gelfand DH, Snisky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 282–287. Academic Press, San Diego. [Google Scholar]
- Lumbsch HT, Huhndorf SM. 2010. Myconet Volume . Part One. Outline of Ascomycota – 2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana, Life and Earth Sciences 1: 1–64. [Google Scholar]
- Masclaux F, Guého E, De Hoog GS, et al. 1995. Phylogenetic relationships of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LS rRNA sequences. Journal of Medical and Veterinary Mycology 33: 327–338. [DOI] [PubMed] [Google Scholar]
- Mason-Gamer RJ, Kellogg EA. 1996. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545. [Google Scholar]
- Matsushima T. 1971. Microfungi of the Solomon Islands and Papua-New Guinea. Matsushima, Kobe. [Google Scholar]
- Matsushima T. 1975. Icones Microfungorum a Matsushima Lectorum. Matsushima, Kobe. [Google Scholar]
- Matsushima T. 1993. Matsushima Mycological Memoirs 7: 1–141. [Google Scholar]
- Matsushima T. 1996. Matsushima Mycological Memoirs 9: 1–30. [Google Scholar]
- Mel’nik VA. 2012. Phaeoisaria vietnamensis sp. nov. and Ph. clematidis (Hyphomycetes) from Vietnam. Mycosphere 3: 957–960. [Google Scholar]
- Mhasker DN, Rao VG. 1976. Development of the ascocarp in Epichloë cinerea (Clavicipitaceae). Mycologia 68: 994–1001. [Google Scholar]
- Minoura K, Muroi T. 1978. Some freshwater ascomycetes from Japan. Transactions of the Mycological Society of Japan 19: 129–134. [Google Scholar]
- Minter DW, Holubová-Jechová V. 1981. New or interesting Hyphomycetes on decaying pine litter from Czechoslovakia. Folia Geobotanica et Phytotaxonomica 16: 195–217. [Google Scholar]
- Müller E, Samuels GJ. 1982. Anamorphs of pyrenomycetous Ascomycetes. II. Porosphaerella gen. nov. and its Cordana anamorph. Sydowia 35: 150–154. [Google Scholar]
- Nannfeldt JA. 1975. Stray studies in the Coronophorales (Pyrenomycetes) 4–8. Svensk Botanisk Tidskrift 69: 289–335. [Google Scholar]
- Nylander J. 2008. MrModeltest2 v. 2.3 (Program for selecting DNA substitution models using PAUP*). Evolutionary Biology Centre, Uppsala, Sweden. [Google Scholar]
- Online Auction Color Chart. 2004. The online auction color chart. The new language of color for buyers and sellers. Online Auction Color Chart Company.
- Pfister DH, Harrington FA, Potter D, et al. 1997. Castor, Pollux and life histories of fungi. Mycologia 89: 1–23. [Google Scholar]
- Pinruan U, Sakayaroj J, Jones EBG, et al. 2004. Flammispora gen. nov., a new freshwater ascomycete from decaying palm leaves. Studies in Mycology 50: 381–386. [Google Scholar]
- Rambaut A, Suchard MA, Xie D, et al. 2013. MCMC trace analysis tool version v1.6.0. Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Ranghoo VM. 1998. Phylogeny of freshwater ascomycetes. PhD Thesis. University of Hong Kong. [Google Scholar]
- Ranghoo VM, Hyde KD. 1998. Ascolacicola aquatica gen. et sp. nov. and a new species of Ascotaiwania from wood submerged in a reservoir in Hong Kong. Mycologia 90: 1055–1062. [Google Scholar]
- Rappaz F. 1992. Phomatospora berkeleyi, P. arenaria and their Sporothrix anamorphs. Mycotaxon 45: 323–330. [Google Scholar]
- Read SJ, Jones EBG, Moss ST. 1993. Taxonomic studies of marine Ascomycotina: ultrastructure of the asci, ascospores and appendages of Savoryella species. Canadian Journal of Botany 71: 273–283. [Google Scholar]
- Réblová M. 1999. Studies in Chaetosphaeria sensu lato I. The genera Chaetosphaerella and Tengiomyces gen. nov. of the Helminthosphaeriaceae. Mycotaxon 70: 387–420. [Google Scholar]
- Réblová M. 2006. Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia 98: 68–93. [DOI] [PubMed] [Google Scholar]
- Réblová M. 2009. Teleomorph of Rhodoveronaea (Sordariomycetidae) discovered and re-evaluation of Pleurophragmium. Fungal Diversity 36: 129–139. [Google Scholar]
- Réblová M, Fournier J, Štěpánek V. 2015. Pisorisporiales, a new order of aquatic and terrestrial fungi for Achroceratosphaeria and Pisorisporium gen. nov. in the Sordariomycetes. Persoonia 34: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M, Gams W, Seifert KA. 2011. Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology 68: 163–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M, Réblová K. 2013. RNA secondary structure, an important bioinformatics tool to enhance multiple sequence alignment: a case study (Sordariomycetes, Fungi). Mycological Progress 12: 305–319. [Google Scholar]
- Réblová M, Seifert KA. 2004. Conioscyphascus, a new ascomycetous genus for holomorphs with Conioscypha anamorphs. Studies in Mycology 50: 95–108. [Google Scholar]
- Réblová M, Seifert KA. 2008. A new species of Chaetosphaeria with Menispora ciliata and phialophora-like anamorphs. Fungal Diversity 29: 99–105. [Google Scholar]
- Réblová M, Seifert KA. 2011. Discovery of the teleomorph of the hyphomycete, Sterigmatobotrys macrocarpa, and epitypification of the genus to holomorphic status. Studies in Mycology 68: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M, Seifert KA, Fournier J, et al. 2012. Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104: 1299–1314. [DOI] [PubMed] [Google Scholar]
- Réblová M, Seifert KA, White GP. 2006. Chaetosphaeria tortuosa, the newly discovered teleomorph of Menispora tortuosa, with a key to known Menispora species. Mycological Research 110: 104–109. [DOI] [PubMed] [Google Scholar]
- Réblová M, Untereiner WA, Réblová K. 2013. Novel evolutionary lineages revealed in the Chaetothyriales (Fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. PLoS ONE 8, 5: e63547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakayaroj J, Pang KL, Jones EBG. 2011. Multi-gene phylogeny of the Halosphaeriaceae: its ordinal status, relationships between genera and morphological character evolution. Fungal Diversity 46: 87–109. [Google Scholar]
- Samuels GJ. 1973. Perithecial development in Hypomyces aurantius. American Journal of Botany 60: 268–276. [Google Scholar]
- Samuels GJ, Blackwell M. 2001, ‘2000’. Pyrenomycetes. In: McLaughlin DJ, McLaughlin EG, Lemke PA. (eds), The mycota. Vol. VII, part A. Systematics and evolution: 221–255. Springer-Verlag, Berlin. [Google Scholar]
- Samuels GJ, Müller E. 1978. Life-history studies of Brazilian Ascomycetes. 1. Two new genera of Sphaeriaceae having, respectively, Sporoschisma-like and Codinaea anamorphs. Sydowia 31: 126–136. [Google Scholar]
- Schoch CL, Sung G-H, Volkmann-Kohlmeyer B, et al. 2007. Marine fungal lineages in the Hypocreomycetidae. Mycological Research 111: 154–162. [DOI] [PubMed] [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9: 1–997. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands. [Google Scholar]
- Shearer CA. 1973. Fungi of Chesapeake Bay and its tributaries. II. The genus Conioscypha. Mycologia 65: 128–136. [Google Scholar]
- Shearer CA, Crane JL. 1971. Fungi of the Chesapeake Bay and its tributaries. I. Patuxent River. Mycologia 63: 237–260. [Google Scholar]
- Shearer CA, Motta JJ. 1973. Ultrastructure and conidiogenesis in Conioscypha (Hyphomycetes). Canadian Journal of Botany 51: 1747–1751. [Google Scholar]
- Shearer CA, Raja HA, Miller AN, et al. 2009. The molecular phylogeny of freshwater Dothideomycetes. Studies in Mycology 64: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan A, Alcorn JL. 2002. Australiasca queenslandica gen. et sp. nov. (Chaetosphaeriaceae: Ascomycota) and its anamorph Dischloridium camelliae sp. nov. from Australia. Australian Systematic Botany 15: 741–747. [Google Scholar]
- Sivanesan A, Chang HS. 1992. Ascotaiwania, a new amphisphaeriaceous ascomycete genus on wood from Taiwan. Mycological Research 96: 481–484. [Google Scholar]
- Sivichai S, Hywel-Jones N, Jones EBG. 1998. Lignicolous freshwater Ascomycota from Thailand: 1. Ascotaiwania sawadae and its anamorph state Monotosporella. Mycoscience 39: 307–311. [Google Scholar]
- Spatafora JW, Johnson D, Sung G-H, et al. 2007, ‘2006’. A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia 98: 1020–1030. [Google Scholar]
- Spatafora JW, Volkmann-Kohlmeyer B, Kohlmeyer J. 1998. Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). American Journal of Botany 85: 1569–1580. [PubMed] [Google Scholar]
- Sri-indrasutdhi V, Boonyuen N, Suetrong S, et al. 2010. Wood-inhabiting freshwater fungi from Thailand: Ascothailandia grenadoidia gen. et sp. nov., Canalisporium grenadoidia sp. nov. with a key to Canalisporium species (Sordariomycetes, Ascomycota). Mycoscience 51: 411–420. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. 2005. RaxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21: 456–463. [DOI] [PubMed] [Google Scholar]
- Sutton BC. 1973. Hyphomycetes from Manitoba and Saskatchewan, Canada. Mycological Papers 132: 1–143. [Google Scholar]
- Sutton DA, Slifkin M, Yakulis R, et al. 1998. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): Criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. Journal of Clinical Microbiology 36: 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CKM, Berbee ML. 2006. Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Molecular Phylogenetics and Evolution 39: 587–597. [DOI] [PubMed] [Google Scholar]
- Tsui CKM, Hodgkiss IJ, Hyde KD. 2003. Three new species of Aquaticola (Ascomycetes) from tropical freshwater habitats. Nova Hedwigia 77: 161–168. [Google Scholar]
- Tsui CKM, Ranghoo VM, Hodgkiss IJ, et al. 2002. Three new species of Annulatascus (Ascomycetes) from Hong Kong freshwater habitats. Mycoscience 43: 383–389. [Google Scholar]
- Udagawa SI, Toyazaki N. 1983. A new species of Conioscypha. Mycotaxon 18: 131–137. [Google Scholar]
- Von Höhnel FXR. 1904. Mykologische Fragmente. Annales Mycologici 2: 38–60. [Google Scholar]
- Von Höhnel FXR. 1909. Fragmente zur Mykologie (VI. Mitteilung, Nr. 182 bis 288). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien. Mathematisch-Naturwissenschaftliche Klasse, Abt. 1, 118: 275–452. [Google Scholar]
- Watanabe T. 1989. Soil fungal flora in Hachijo-jima island. Transactions of Mycological Society of Japan 30: 427–435. [Google Scholar]
- Watanabe T. 1992. Taeniolella phialosperma sp. nov. from Japan. Mycologia 84: 478–483. [Google Scholar]
- Zare R, Gams W, Starink-Willemse M, et al. 2007. Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwigia 85: 463–489. [Google Scholar]
- Zelski SE, Raja HA, Miller AN, et al. 2011. Chaetorostrum quincemilensis, gen. et sp. nov., a new freshwater ascomycete and its Taeniolella-like anamorph from Peru. Mycosphere 2: 593–600. [Google Scholar]
- Zelski SE, Raja HA, Miller AN, et al. 2014. Conioscypha peruviana sp. nov., its phylogenetic placement based on 28S rRNA gene, and a report of Conioscypha gracilis comb. nov. from Peru. Mycoscience 56: 319–325. [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, et al. 2007, ‘2006’. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076–1108. [DOI] [PubMed] [Google Scholar]


