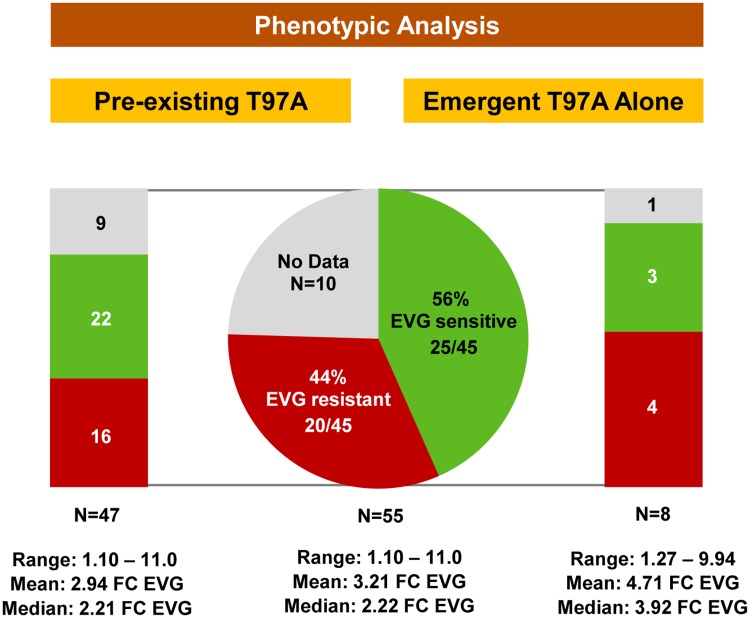
Fig 3. Pre- and on-treatment incidence of HIV-1 infected patients with T97A-based phenotypic EVG resistance.
Grey indicates no data; black indicates excluded data, green indicates EVG susceptibility (i.e., EVG sensitive), red indicates reduced EVG susceptibility (i.e., EVG resistant). Left panel: Forty-seven patients had pre-existing T97A: 16 patients (9 enrolled on INSTI: 1 TE [on EVG+TDF+DRV/r] and 8 TN [on EVG/COBI/FTC/TDF]) with reduced EVG susceptibility, 22 patients (9 enrolled on INSTI: 2 TE [on EVG/r+TDF+DRV] and 7 TN [on EVG/COBI/FTC/TDF]) with EVG sensitivity, and 9 patients with no phenotypic data. Right panel: Eight patients (TE = 7; TN = 1) had emergent T97A alone (i.e., in the absence of primary INSTI RAMs): 4 patients (4 TE [2 on RAL+ABC+LPV/r, 1 on EVG/r+MVC+DRV, and 1 on EVG/r+TDF+DRV]) with reduced EVG susceptibility, 3 patients (3 TE [1 on EVG/r+TDF+DRV, 1 on EVG/r+FTC/TDF+LPV, and 1 on EVG/r+ETR+LPV]) with EVG sensitivity including 1 patient (1 TE [on EVG/r+ETR+LPV]) that withdrew consent and discontinued study drug at Week 144 and was excluded from treatment outcome and study-specific resistance analysis (see Methods), and 1 patient (1 TN [on EVG/COBI/FTC/TDF]) with no phenotypic data. The proportion of patients with reduced EVG susceptibility was low and similar between pre- and on-treatment T97A populations (P = 0.682).