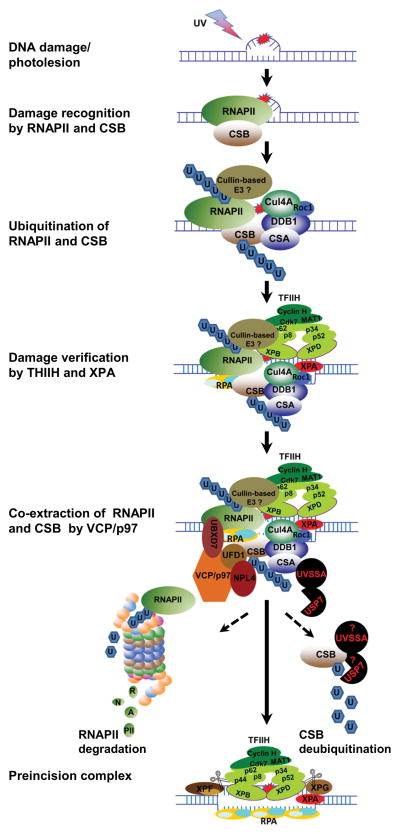
Figure 3.
Schematic model depicting the role of VCP/p97 in the assembly of preincision complex during TC-NER. When elongating RNAPII encounters a transcription-blocking lesion, it is transiently installed by the lesion. The installed RNAPII stabilizes the interaction between RNAPII elongation machinery and preexisting CSB or recruits CSB de novo. The transiently stabilized RNAPII elongation machinery stimulates the formation of a functional Cullin-based Elongin A ubiquitin ligase complex or recruits a RNAPII E3 ubiquitin ligase. Simultaneously, CRL4CSA is recruited to the arrested machinery in a CSB-dependent manner. Consequently, RNAPII and CSB are independently ubiquitinated. The ubiquitin conjugates recruit VCP/p97 complex, which may dynamically interact with activated CRL4. Next, TFIIH and XPA arrive and replace RNAPII when RNAPII-CSB is co-extracted by VCP/p97 complex. Finally, the arrival of XPG and XPF as well as RPA completes the formation of preincision complex. The ubiquitinated CSB undergoes deubiquitination by USP7, whereas ubiquitinated RNAPII is degraded by proteasome.