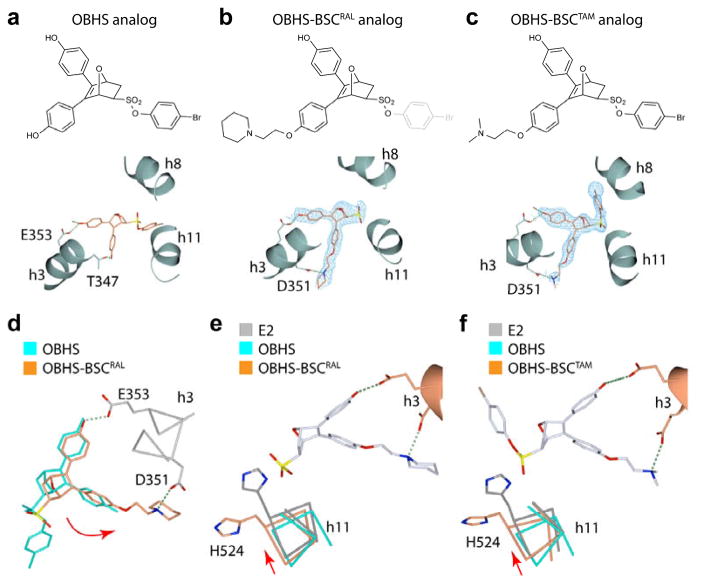
Figure 4.
The SERM-derived BSC alters the OBHS-binding orientation. (a–c) Crystal structures of ERα LBDs showing the binding orientations of (a) an OBHS analog (PDB 4ZNW), and OBHS-BSC analogs with either (b) a raloxifene-derived side chain (PDB 5TN9), or (c) a tamoxifen-derived side chain (PDB 5TNB). The chemical structure of the bound ligand is shown above each panel. The 2Fo-Fc electron density and Fo-Fc difference maps of OBHS-BSC analogs were contoured at 1.0 σ and 3.0 σ, respectively. (d) The BSC-mediated h-bond with Asp351 rotates the oxabicyclic ligand core (curved red arrow). The OBHS- and OBHS-BSC-bound LBDs in panels a and b were superposed. (e–f). BSC-induced rotation of the ligand core repositions h11. The OBHS- and OBHS-BSC-bound LBDs in panels a–c were superposed on the E2-bound LBD (PDB 3UUD).