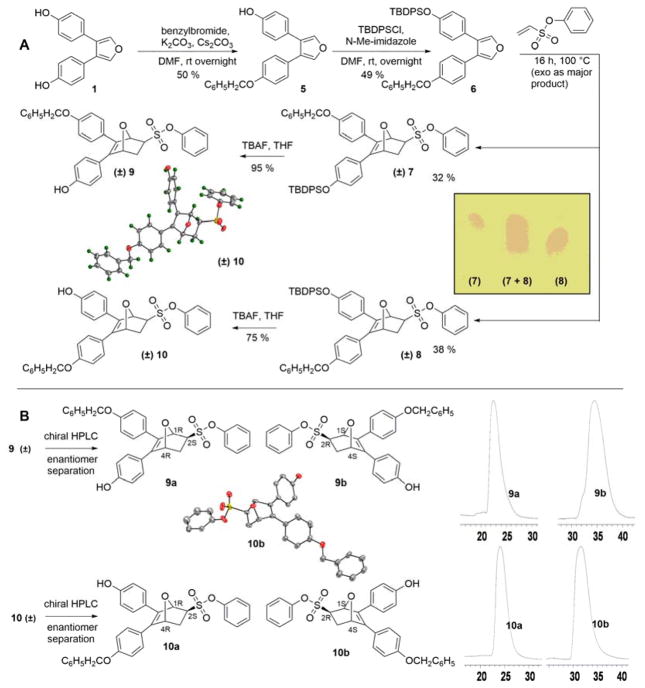
Scheme 2.
A Synthesis and separation of regioisomers of monophenol protected OBHS derivatives. (Insets: ORTEP diagram of compound 10 as a racemate showing the distal arrangement between the benzyl ether and phenyl sulfonate. TLC analysis of the migration of the regioisomeric benzyl TBDPS bis-ethers, 7 and 8 as racemates.) B Separation of enantiomers by chiral HPLC. The HPLC traces of pure enantiomers are also shown (scale in minutes). The assignment of absolute stereochemistry to compounds 9a and 9b are made by analogy to 10a and 10b, respectively (see text for details). (Insets: ORTEP diagram of a pure enantiomer of OBHS regioisomer 10 (10b, with 1S,2R,4S stereochemistry). Preparative HPLC analysis of the elution of the enantiomers of the proximal regioisomers (9a and 9b) and the distal regioisomers (10a and 10b)).