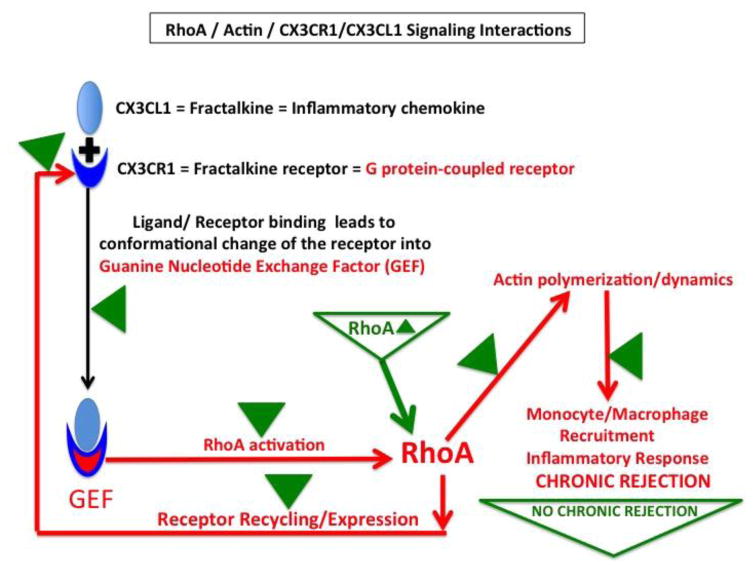
Figure 8. Hypothetical model of the effect of RhoA, actin and CX3CR1/CX3CL1 interactions on development of chronic rejection of the allograft.
The fractalkine (CX3CL1) is an inflammatory chemokine abundantly produced by vasculature endothelium. Fractalkine receptor (CX3CR1)-expressed on the surface of monocytes/macrophages is a G protein-coupled receptor. It is known that upon binding to fractalkine the CX3CR1 changes conformation and becomes GEF, which activates RhoA. This induces changes in actin polymerization allowing monocyte/macrophages to enter the allograft. Once in the allograft, they mount inflammatory response, neointimal hyperplasia and chronic rejection. Simultaneously, activation of RhoA and proper actin organization/dynamics control CX3CR1 recycling. Correct receptor recycling controls CX3CR1 mRNA and protein expression levels. Upon RhoA deletion (marked by green Δ) all above interactions and signaling pathways and their effects are disrupted (green Δ) leading to inhibition of chronic rejection.