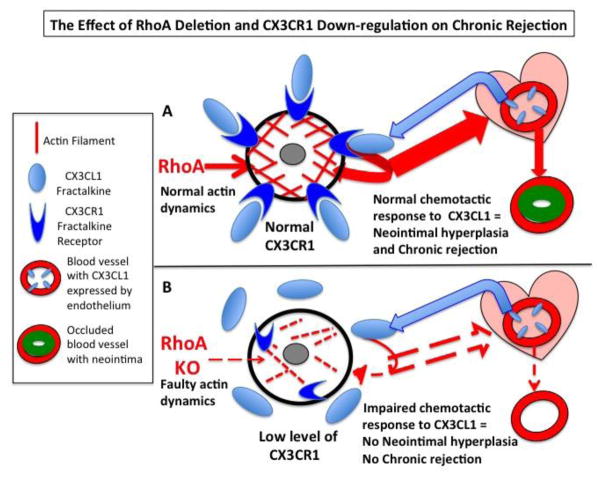
Figure 9. Hypothetical model of the effect of monocyte/macrophage-targeted RhoA deletion on neointima hyperplasia and chronic rejection.
(A) Normal (undeleted) monocytes and macrophages have normal actin organization/dynamics and express high level of CX3CR1 receptor. In response to transplantation, the allograft and the endothelium of its vasculature produce fractalkine chemokine (CX3CL1), which is a ligand of CX3CR1 receptor. Binding of fractalkine to its receptor induces chemotactic response and recruits monocytes/macrophages toward the allograft. While in the allograft, the monocytes differentiate into macrophages and together with co-recruited macrophages mount inflammatory response and cause over-proliferation of smooth muscle cells in the vascular walls. This in turn leads to the neointimal hyperplasia, occlusion of arteries and chronic rejection of the allograft. (B) RhoA deleted monocytes and macrophages have abnormal actin organization/dynamics, which lowers expression of CX3CR1 receptors. The CX3CR1-deficient monocytes/macrophages are unable to respond to fractalkine signaling to be recruited to the allograft. Thus, only very low number of monocytes/macrophages still expressing high level of CX3CR1 is recruited to the allograft. The very low number of these monocytes/macrophages results in lower inflammatory response, lower neointimal hyperplasia and diminished chronic rejection of the allograft.