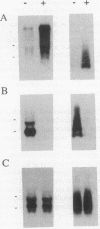
Abstract
A gene expression screen identifies mRNAs that differ in abundance between two mRNA mixtures by a subtractive hybridization method. The two mRNA populations are converted to double-stranded cDNAs, fragmented, and ligated to linkers for polymerase chain reaction (PCR) amplification. The multiple cDNA fragments isolated from any given gene can be treated as alleles in a genetic screen. Probability analysis of the frequency with which multiple alleles are found provides an estimation of the total number of up- and down-regulated genes. We have applied this method to genes that are differentially expressed in amphibian tadpole tail tissue in the first 24 hr after thyroid hormone treatment, which ultimately induces tail resorption. We estimate that there are about 30 up-regulated genes; 16 have been isolated.
Full text
PDF
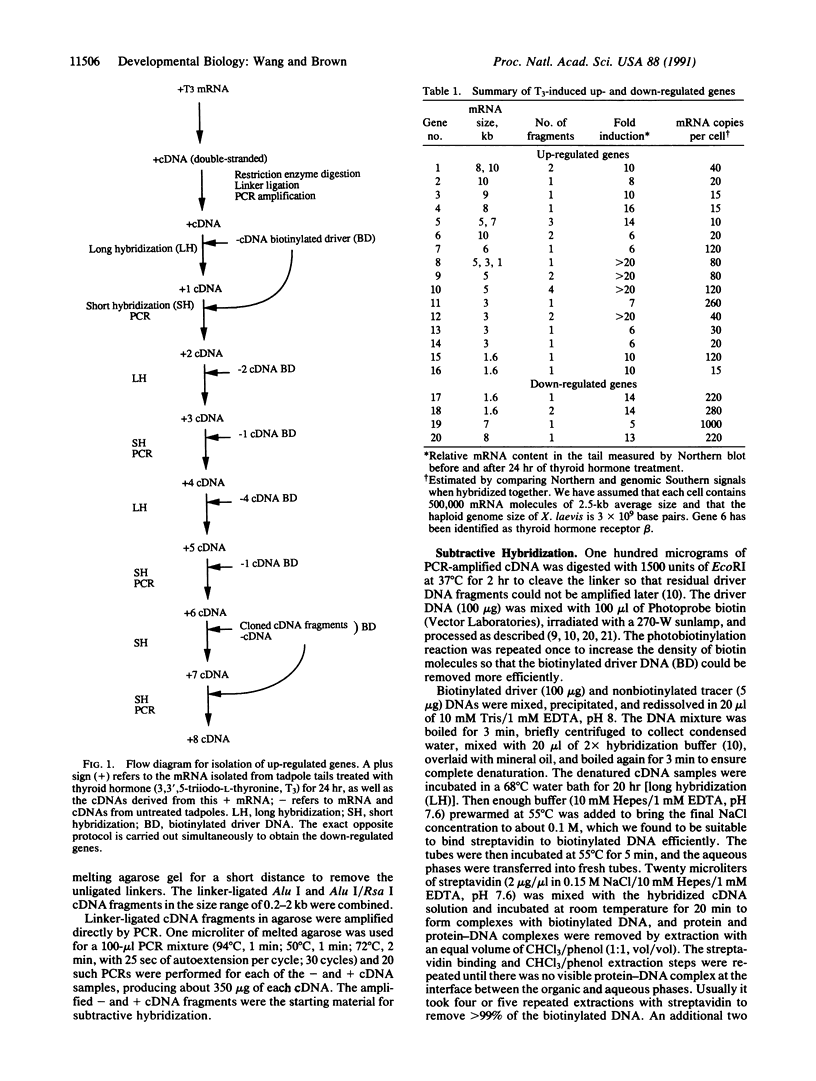
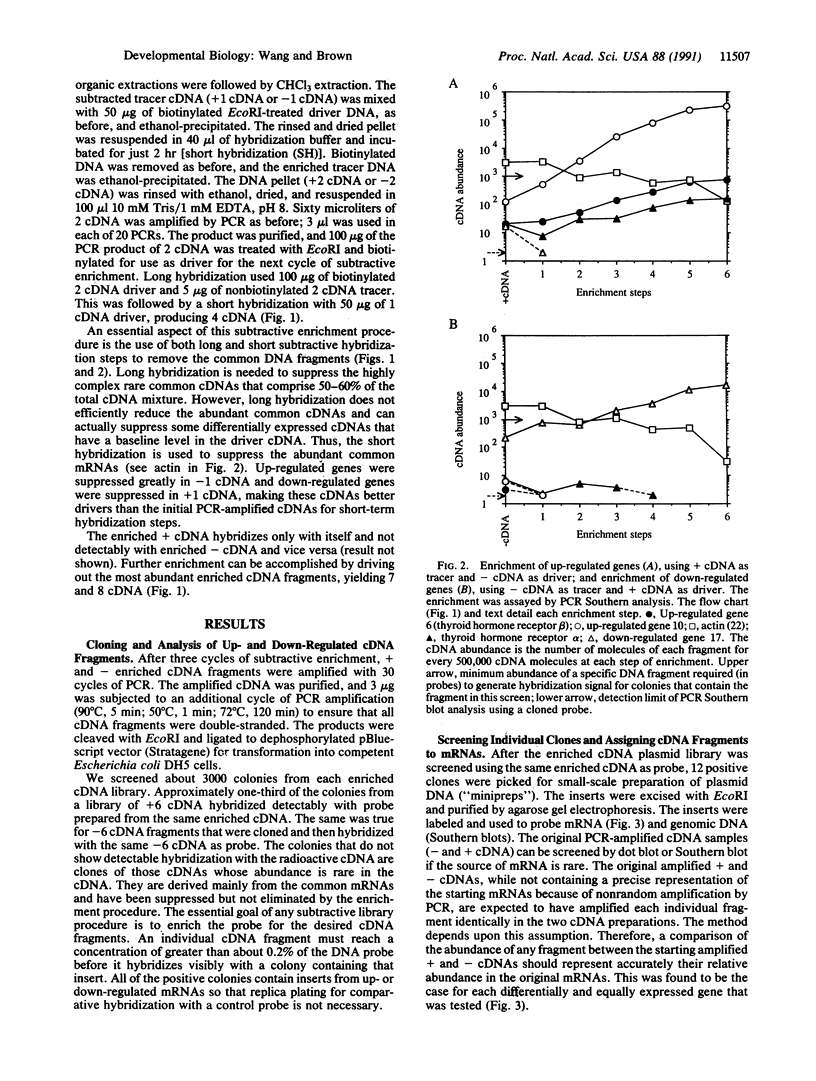
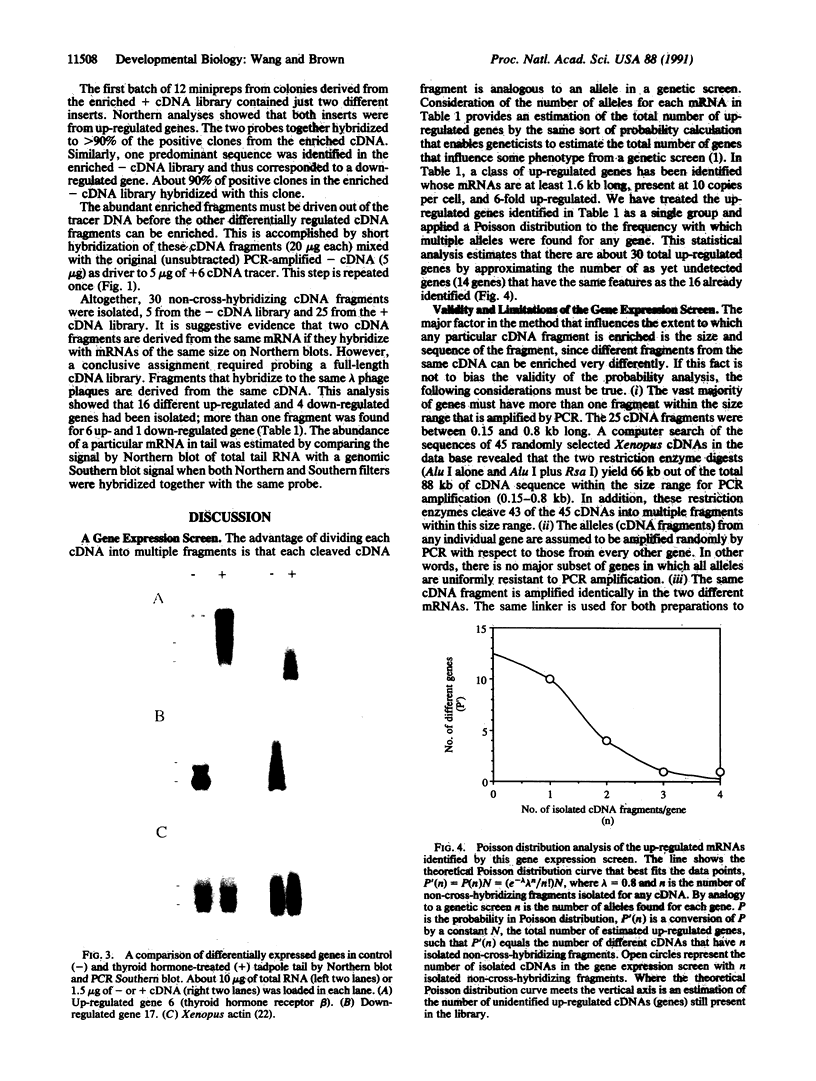

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Dinauer M. C. Library subtraction of in vitro cDNA libraries to identify differentially expressed genes in scrapie infection. Nucleic Acids Res. 1990 May 11;18(9):2789–2792. doi: 10.1093/nar/18.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargnoli J., Holbrook N. J., Fornace A. J., Jr Low-ratio hybridization subtraction. Anal Biochem. 1990 Jun;187(2):364–373. doi: 10.1016/0003-2697(90)90471-k. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather E. L., Alt F. W., Bothwell A. L., Baltimore D., Koshland M. E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981 Feb;23(2):369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Mohler J. D. Developmental genetics of the Drosophila egg. I. Identification of 59 sex-linked cistrons with maternal effects on embryonic development. Genetics. 1977 Feb;85(2):259–272. doi: 10.1093/genetics/85.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Brennan S., Dathan N., Fairman S., Gurdon J. B. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984 Oct 25;311(5988):716–721. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Brice A. E., Ciaranello R. D., Denney D., Porteus M. H., Usdin T. B. Subtractive hybridization system using single-stranded phagemids with directional inserts. Nucleic Acids Res. 1990 Aug 25;18(16):4833–4842. doi: 10.1093/nar/18.16.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Dawid I. B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983 Oct 14;222(4620):135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- Sive H. L., St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucleic Acids Res. 1988 Nov 25;16(22):10937–10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcher A. A., Torres A. R., Ward D. C. Selective enrichment of specific DNA, cDNA and RNA sequences using biotinylated probes, avidin and copper-chelate agarose. Nucleic Acids Res. 1986 Dec 22;14(24):10027–10044. doi: 10.1093/nar/14.24.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland I., Bolger G., Asouline G., Wigler M. A method for difference cloning: gene amplification following subtractive hybridization. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2720–2724. doi: 10.1073/pnas.87.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y., Brown D. D. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990 Nov;4(11):1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]