Abstract
Background
Oral endocrine therapy (OET) such as Tamoxifen or aromatase inhibitors reduces recurrence and mortality for the 75% of breast cancer survivors (BCS) diagnosed with estrogen-receptor positive breast cancer. Because many BCS decide not take OET as recommended due to side effects, understanding BCS's decisional supports and needs is foundational to supporting quality OET decision making about whether or not to adhere to OET.
Objective
To examine literature pertaining to OET non-adherence and side effects using the Ottawa Decision Support Framework categories of decisional supports and decisional needs as these factors potentially influence OET use.
Methods
A systematic literature search was performed in PubMed and CINAHL using combined search terms “aromatase inhibitors and adherence” and “tamoxifen and adherence.” Studies that did not meet criteria were excluded. Relevant data from 25 publications was extracted into tables and reviewed by two authors.
Results
Findings identified: the impact of side effects on OET non-adherence; an absence of decisional supports provided to or available for BCS who are experiencing OET side effects; and the likelihood of unmet decisional needs related to OET.
Conclusions
Side effects contribute to BCS's decisions to stop OET, yet there has been little investigation of the process through which that occurs. This review serves as a call to action for providers to provide support to BCS experiencing OET side effects and facing decisions related to non-adherence.
Implications for Practice
Findings suggest BCS prescribed OET have unmet decisional needs and more decisional supports are needed for BCS experiencing OET side effects.
Keywords: adherence/non-adherence, breast cancer, decision making, OET, tamoxifen, side effects, aromatase inhibitors
Oral endocrine therapy (OET) is standard therapy for estrogen receptor-positive (ER+) breast cancer.1 An estimated 75% of women with breast cancer receive a recommendation for life-saving OET such as tamoxifen or aromatase inhibitors.1 OET is prescribed for ER+ breast cancer to prevent recurrence by blocking certain hormones that fuel cancer growth.
The approach to oral endocrine therapy treatment in BCS with ER+ breast cancer depends on whether or not a woman is in menopause. Tamoxifen is prescribed to pre-, peri, or postmenopausal women and has been shown to decrease breast cancer recurrence by 41% and mortality by 34%.2, 3 Aromatase inhibitors (AI)s, prescribed only for postmenopausal women, have been shown to reduce recurrence by 30%-41% and metastasis by 16%-18%, with mortality rate reductions similar to tamoxifen. 2, 3 4As a class, the AIs have consistently been shown to improve outcomes for postmenopausal women with hormone receptor-positive breast cancer compared with tamoxifen.4 Each agent is taken on a daily basis for the duration of a minimum of 5 years, sometimes longer.
Despite the benefits of OET for BCS diagnosed with ER+ breast cancer, many BCS decide not to take their OET as recommended.7-9,13,17,30 The decision to take OET is not a single event decision, but a complex process that occurs over time as a series of one time daily decisions or twice daily decisions. Studies show that 30-50% of BCS who initiate therapy are not adherent to daily or twice-daily pill ingestion, and alarmingly 70% prematurely stop the therapy before the end of the once recommended 5-year period.2, 3 More recently, trials suggest that 10 years of tamoxifen are better than 5 years and that a program of extended adjuvant therapy of tamoxifen for 5 years followed by aromatase inhibitor for 5 years is effective for suitable candidates. 4This new recommendation causes more concern regarding the 70% early termination rates seen with a 5 year course of therapy.
Understanding a BCS's decisional supports (e.g. any support given to meet an identified decisional need) and decisional needs (e.g. any need a person may have that results in difficulty making a quality decision) is important to help facilitate adherence to OET, particularly when side effects are experienced.15 Tamoxifen side effects include hot flashes, weight gain, and loss of libido, and less commonly thromboembolic disease or endometrial pathologies.4-9 Aromatase inhibitor side effects include hot flashes, arthralgia, increased fractures, rash, and gastrointestinal upset.4, 5, 10-14 Understanding the decisional needs and support is a first step in creating a patient centered intervention to increase the percentage of BCS that correctly use this potentially life-saving treatment.
Purpose and Aims
The purpose of this review was to examine literature pertaining to OET non-adherence and side effects using the Ottawa Decision Support Framework categories of decisional supports and decisional needs as requisites for quality decision making. Aims were to use the available literature to (1) summarize the general nature of the studies, (2) summarize the link between prevalence of non-adherence and side effects, (3) summarize details of BCS's decisional supports, and (4) summarize thematic categories of BCS's unmet decisional needs.
Conceptual Framework
The Ottawa Decision Support Framework was the conceptual framework for this study. The framework suggests that quality decisions result when decisional needs (e.g. knowledge, expectations, values) are understood and appropriate decisional supports (e.g. coaching, counseling, providing facts and probabilities) are provided.15 Decisional support is defined as any support that is given to meet an identified decisional need.15 The goal of decisional support is to address modifiable determinants of decision making that are suboptimal. These determinants can include inadequate knowledge, unrealistic expectations, unclear values, unclear norms, unwanted pressure, inadequate support, and inadequate personal and external resources to make the decision.4, 15, 16 Decisional needs are defined as any need a person may have that results in a difficulty to make a decision.15 Decisional needs are based on the knowledge, degree of certainty, expectations, and values one may have regarding the decision to be made.15, 16
Methods and Search Strategy
A systematic literature search was performed in PubMed and CINAHL. The PubMed database was selected because biomedical topics and the sciences are the primary foci of articles contained in this database, and these content areas directly related to the topic for this review. In addition, PubMed includes all articles indexed in MEDLINE.37 CINAHL was selected instead of OVID for its coverage of full-text nursing medical journals published by many different publishers. OVID searches are limited to articles published only by OVID and its publishing partners.38 Only peer reviewed articles were included in the review so PROQUEST or other dissertation search engines were not included. The search strategy for PubMED and CINAHL databases combined the search terms “aromatase inhibitors and adherence” and “tamoxifen and adherence.” In order to maximize inclusion, study type and publication date were not limited in the search strategy and articles including all factors associated with OET non-adherence (not just side effects as a single factor) were included. In addition, reference lists of identified review articles were hand searched to identify potentially relevant additional articles. First, titles and abstracts were screened. Second, the full texts of all potentially relevant articles were obtained and read to determine suitability for inclusion. Articles were identified for inclusion by the primary author according to predetermined criteria and then verified by a second reviewer.
Inclusion and exclusion criteria
To be eligible for this review, manuscripts had to meet the following inclusion criteria: (1) study population of adult females with a diagnosis of breast cancer, (2) intake of tamoxifen or aromatase inhibitors, (3) quantitative or qualitative analyses between medication and adherence (e.g. reported side effects attributed to non-adherence), and (4) full-length, original research. All types and stages of breast cancer were included. Excluded were articles that were (1) non-English language, (2) focused solely on reporting only adherence rates and not including factors contributing to non-adherence, (3) reviews, or (4) editorials, opinion papers, or abstracts.
Data extraction
Data were extracted and organized into 4 separate tables and described below. All extracted data were verified by a second reviewer. Table 1 in analysis contained a general overview of the study characteristics including author, publication year, country where the study occurred, study design, length of study, cancer stage of participants, sample size, and the class of medication (tamoxifen or aromatase inhibitor). Table 2 focuses on rates of non-adherence to the OET assessed in each study, the prevalence of side effects reported, and whether or not side effects were reported as a reason for non-adherence. Data extracted into Table 3 focused on decisional support participants reportedly received when receiving the OET prescription or at follow-up visits during recommended treatment. Using categories from the ODSF, the table delineates the type, source, timing, and content of provided support. Table 4 focuses on decisional needs. Identified needs are grouped according to four thematic categories that emerged from the available data within the articles: (1) regimen (not understanding timing, dose, or duration of OET), (2) beliefs about benefits and risks (OET being unhelpful, not necessary, or other negative or neutral beliefs about OET), (3) inadequate information (insufficient or confusing information, inadequate knowledge of side effects, or inadequate knowledge of tumor hormone status), and (4) no one to ask questions (inadequate support to gather information). For studies that contained no detailed information, decisional needs were marked as “not reported.”
Table 1. Study Characteristics.
| Author, year, country | Study Design and Length | Sample | Medication | |||
|---|---|---|---|---|---|---|
| Cancer Stage | N | Tamoxifen | Aromatase Inhibitor | |||
| Aiello Bowles et al., 2012, USA | Cross-sectional survey | 3 mos. | 1,2 | 538 | X | X |
| Atkins & Fallowfield, 2006, UK | Cross-sectional survey | NR | 131 | X | X | |
| Bell et al., 2013, Australia | Prospective longitudinal survey | 4 yrs. | 1,>1 | 1193 | X | X |
| Boonstra et al., 2013, Netherlands | Cross-sectional observational study | 3 mos. | 1,2,3 | 57 | X | |
| Bramwell et al., 2009, Canada | Randomized, placebo-controlled trial | 12 yrs. | 1,2,3 | 672 | X | |
| Chim et al., 2013, USA | Cross-sectional survey | 18 mos. | 1,2,3 | 437 | X | |
| Cluz et al., 2012, France | Prospective longitudinal survey | 3.5 yrs. | 1,2,3 | 196 | x | |
| Demissie et al., 2001, USA | Prospective longitudinal survey | 3 yrs. | 1,2 | 292 | X | |
| Fink et al., 2004, USA | Prospective longitudinal survey | 3 yrs. | 1,2,3a | 597 | X | |
| Grunfield, 2005, UK | Cross-sectional survey | NR | NR | 110 | X | |
| Harrow, A. et al., 2014, UK | Qualitative semi-structured interviews | 6 mos. | NR | 30 | X | X |
| Henry et al., 2012, USA | Randomized clinical trial | 4 yrs. | 0,1,2,3 | 503 | X | |
| Kahn et al., 2007, USA | Prospective Cohort Study | 4 yrs. | 1,2,3 | 881 | X | |
| Kemp, 2014, Australia | Observational | 5 yrs. | All | 1531 | X | X |
| Kirk & Hudis, 2008, USA | Online survey | 6 yrs. | All | 328 | X | X |
| Kyvernitakis et al., 2014, Germany | Randomized clinical trial | 2 yrs. | 1,2,3 | 180 | X | |
| Lash et al., 2006, USA | Prospective longitudinal survey | 3 yrs. | I,2,3a | 462 | X | |
| Mao et al., 2013, USA | Content analysis | NR | All | 25256 posts | X | |
| Oberguggenberger et al., 2011, Austria | Cross-sectional survey | 5 yrs. | 1,2,3 | 280 | X | |
| Owusu et al., 2011, USA | Prospective cohort study | 5 yrs. | 1-2b | 961 | X | |
| Pellegrini et al., 2010, France | Qualitative Grounded theory study | NR | NR | 34 | X | |
| Schover et al., 2014, USA | Cross-sectional survey | single mailed survey | 1-2a | 129 | X | |
| Simon et al., 2014, Canada | Cross-sectional survey | 6 mos. | 0-4 | 161 | X | X |
| Stanton, Petrie & Partridge, 2014, USA | Cross-sectional survey | 2 weeks | 0-4 | 1465 | X | X |
| Wouters et al., 2013, Netherlands | Qualitative Focus Groups and individual interviews | <1 year post-OET completion | NR | 37 | X | X |
Abbreviations: mos., months; NR, not reported; OET, oral endocrine therapy; X, medication included in study; yrs., years.
Table 2. Side effects.
| Authors | Prevalence of Non adherence (stopping OET prior to 5 years) | Prevalence of Side Effects | Side effect Noted as Reason for Non-adherence | |||
|---|---|---|---|---|---|---|
| TAM | AI | Both | TAM | AI | ||
| Quantitative Studies | ||||||
| Aiello Bowles et al. | 43.9% (n=43) | 22.4% (n=22) | 33.7% (n=33) | 66.70% (n=32) ++ | 59.10%(n=39) | X |
| Atkins & Fallowfield | 54% (n=39) | 61% (n=22) | NR | NR | X | |
| Bell et al. | 7.30% N=88 | 5.80% N=69 | NR | a | a | X |
| Boonstra et al. | 31% | 74% | NR | |||
| Bramwell et al | 26% (n=173) | 8% (n=29) | X | |||
| Chim et al. | 11% (n=47) | 82% (n=358) | X | |||
| Cluze et al. | 40% (n=27) | 42% (n=5)aa | 47% (n=92) | aa | X | |
| Demissie et al. | 15% (n=26) | 63% (n=104) | X | |||
| Fink et al. | 17% (n=88) | 45% (n=271) | NR | |||
| Grunfield | 13% (n=13) | 46% (n=6) | X | |||
| Henry et al. | 43% (n=216) | 33% (n=163) | X | |||
| Kahn et al. | 21% (n=185) | 21% (n=185) | X | |||
| Kemp | 58% (n=888) | NR | X | |||
| Kirk & Hudis | NR | NR | 17% (n=53) | 69.80% (n=37) | X | |
| Kyvernitakis et al. | 22% (n=40) | 100% (n=159) | X | |||
| Lash et al. | 31% (n=143) | 49% (n=227) | X | |||
| Mao et al. | 13% (n=110) | 18.2% (n=4,596 posts) | X | |||
| Owusu et al. | 46% (n=442) | NR | X | |||
| Oberguggenberger et al. | NR | 59.6% (n=167) | X | |||
| Schover et al. | 15.5% (n=20) | 79% (n=53) | X | |||
| Stanton, Petrie, & Partridge | 3% (n=44) | 48% (n=326) | X | |||
| Qualitative Studies | ||||||
| Harrow, A. et al. | 10% (n=3) | NR | NR | X | ||
| Mao et al. | 13% (n=110) | 18.2% (n=4,596 posts) | X | |||
| Pellegrini et al. | 18% (n=6) | NR | X | |||
| Simon et al. | NR | NR | 6% (n=7) | 6% (n=7) | X | |
| Wouters et al. | 19% (n=7) | NR | NR | X | ||
Abbreviations: NR, not reported; TAM, tamoxifen; AI, aromatase inhibitor; X, side effect noted as reason for non-adherence.
some BCS reported more than one side effect, some reported none. All answers were included; prevalence unable to be determined
Rates of interruption of AI reported on larger cohort sample not included in analyses
Table 3. Decisional Support.
| Details Contained in Articles on the Type, Source, Timing, and Content of Messages Given for Decisional Support | ||||
|---|---|---|---|---|
| Author(s) | Type | Source | Timing | Message Content |
| Aiello Bowles et al. | * | * | * | * |
| Atkins & Fallowfield | * | * | * | * |
| Bell et al. | Verbal | Provider | Following RX | * |
| Boonstra et al. | Verbal | Provider | Prior to RX and at followup | Side effects information |
| Bramwell et al | * | * | * | * |
| Chim et al. | * | * | * | * |
| Cluz et al. | Verbal | Provider | Following RX | BCS reported they were not given understandable OET-related information BCS reported they did not consider their information sufficient BCS reported they did not have the opportunity to ask questions at diagnosis |
| Demissie et al. | Print, media, verbal | Books Magazines Television Provider | Following RX | * |
| Fink et al. | * | * | * | * |
| Grunfield | * | * | * | * |
| Harrow, A. et al. | Print, media, verbal | Internet Provider | Following RX | Even though given side effects information, BCS reported not being asked whether or not they were still taking the medication at follow-up visits |
| Henry et al. | * | * | * | * |
| Kahn et al. | Verbal | Provider | Following RX | BCS reported not receiving information about side effects in advance from their provider BCS reported not receiving adequate information from their provider |
| Kemp | * | * | * | * |
| Kirk & Hudis, | Verbal | Provider | Following RX and at follow-up visits | BCS told importance of taking OETs at almost every visit BCS discussed side effects with provider |
| Kyvernitakis et al. | * | * | * | * |
| Lash et al. | * | * | * | * |
| Mao et al. | Media | Internet message boards | Not specified | Side effects information |
| Oberguggenberger et al. | * | * | * | * |
| Owusu et al. | * | * | * | * |
| Pellegrini et al. | Print, media, verbal | Peers, Provider Internet | Following RX | Side effects information described OET as hormone or anti-hormone |
| Schover et al. | Verbal | Provider | Following RX | Side effects information |
| Simon et al. | * | * | * | * |
| Stanton, Petrie, & Partridge | * | * | * | * |
| Wouters et al. | Verbal | Provider | Following RX | Side effects information provided BCS reported they were not given information that taking OET at the same time every day was important BCS reported that the duration of therapy was unclear |
Abbreviations: RX, Prescription;
no information available in article
Table 4. Decisional Needs.
| Author | Regimen (timing, dose, duration) | Beliefs of Benefits & Risks | Inadequate Information | No One to Ask Questions | No Information Reported |
|---|---|---|---|---|---|
| Aiello Bowles et al. | X | ||||
| Atkins & Fallowfield | X | ||||
| Bell et al. | X | ||||
| Boonstra et al. | X | ||||
| Bramwell et al | X | ||||
| Chim et al. | X | ||||
| Cluz et al. | |||||
| Demissie et al. | X | ||||
| Fink et al. | X | ||||
| Grunfield | X | ||||
| Harrow, A. et al. | X | ||||
| Henry et al. | X | ||||
| Kahn et al. | X | ||||
| Kemp | X | ||||
| Kirk & Hudis, | X | ||||
| Kyvernitakis et al. | X | ||||
| Lash et al. | X | ||||
| Mao et al. | X | ||||
| Oberguggenberger et al. | X | ||||
| Owusu et al. | X | ||||
| Pellegrini et al. | X | ||||
| Schover et al. | X | ||||
| Simon et al. | X | ||||
| Stanton, Petrie, & Partridge | X | ||||
| Wouters et al. | X | X |
Abbreviations: X = article described unmet decisional needs within this category
Results
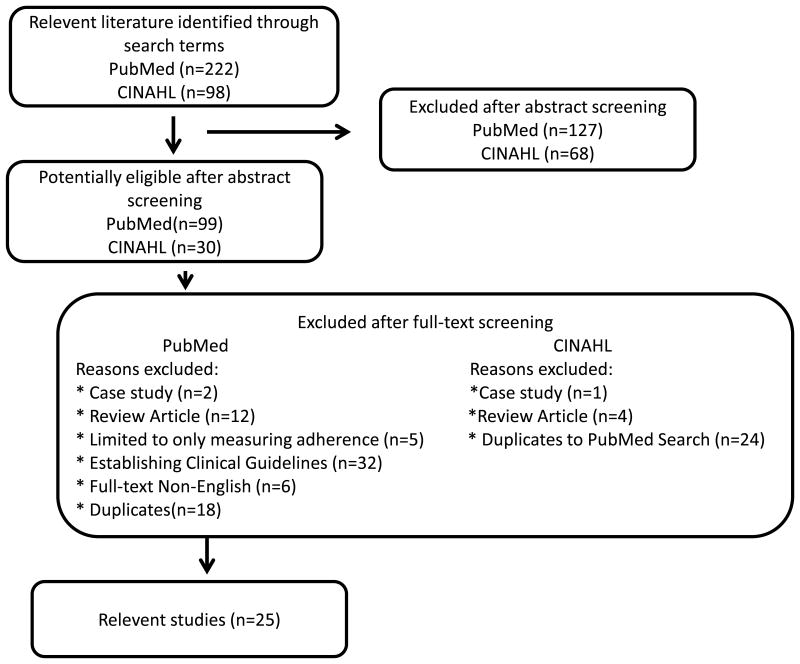
The search in the PubMed electronic database yielded 222 articles. After title and abstract screening, 99 articles were identified as potentially relevant. After removing 18 duplicates, 81 full-text versions were screened in detail. Finally, 24 studies were included. The search in the CINAHL electronic database yielded 98 articles. After title and abstract screening, 24 duplicate articles that were found in the PubMed were excluded and 6 articles were screened in detail. Finally, one additional article from the CINAHL search was included in the review. The manual search and reference check revealed no further relevant publications. The flowchart in the Figure 1 illustrates the selection process.
Figure 1. Flow Diagram.
Characteristics of studies
The characteristics of identified studies are summarized in Table 1. Most articles were published after 2012 (n=13, 54.0%), 5, 6, 10-14, 17-22,36 with publication dates ranging from 2001-2014. The majority of studies were conducted in the United States (n=12, 48.0%),5-7, 9, 12-14, 21, 23-25 used quantitative methods, and reported on data collected using standardized self-report measures. Duration of study time points varied from a single one-time mailing to 12 years, and not all studies reported this information. Stage of breast cancer ranged from 0-IV with not all studies reporting this information. Sample sizes ranged from 30 BCS to 1,531 online posts by BCS. Participant ages ranged from 18 to >85. Class of OET studied was fairly well distributed across the relevant literature with 9 studies (36.0%) including both types of OET, 9 studies (37.5%) reporting on tamoxifen, and 7 studies (29.1%) reporting on aromatase inhibitors.
Prevalence of non-adherence and side effects
Prevalence of non-adherence varied by drug. As shown in Table 2, non-adherence rates to tamoxifen ranged from around 7.3% to 54.0% and to AI ranged from 5.8% to 61.0%. In studies that reported non-adherence rates to overall therapy and not individually by drug adherence rates, rates were reported as 3.0%-58.0%.
Prevalence of side effects also varied by drug. As shown in Table 2, Tamoxifen side effect prevalence ranged from 8.0%-66.7% and AI side effect prevalence ranged from 18.2%-66.7%. In studies that reported prevalence to overall therapy and not individually by drug, side effect prevalence rates ranged from 3.0%-69.8%. Four studies did not provide side effect prevalence rates but did include narrative description on the impact of experiencing side effects on adherence to OET.
Side effects were a reason for non-adherence in 23 of the 25 (92.0%) identified studies. Studies that focused on both tamoxifen and AIs often did not report side effects by drug. Four studies (16.0%) did not include information on specific side effects experienced. In addition, 1 study (4.2%) measured only severity and not type of side effects, 2 studies (8.3%) were specific to a single side effect, and 1 study (4.2%) reported the general experience of side effects. Hot flashes were described in 13 studies (54.2%), joint pains in 8 studies (33.3%), fatigue/loss of energy in 7 studies (29.2%), mood problems in 6 studies (25.0%), sexual dysfunction in 5 studies (20.8%), night sweats in 4 studies (16.7%), and sleep problems in 2 studies (8.3%).
Decisional supports
Details on decisional support were absent in 13 (52.0%) of the identified studies, either because decisional support was not assessed or was not reported as part of the results. As shown in table 3, types and sources of decisional support included verbal information from providers as well as print or media (Internet, magazines, television, books). The time when decisional support was provided or sought by BCS was commonly reported as following initial prescription, but some articles also alluded to support being provided prior to initial prescription and also at follow-up visits. Message content was not always described within the articles. In 4 other studies (16.7%), information was limited to side effects only. In addition, in 4 studies (16.7%), participants specifically described the information they received as being insufficient. Only 1 study (4.2%) included information that BCS were informed of the importance of taking OET at almost every visit and had the opportunity to discuss side effects with their provider.
Decisional needs
Decisional needs are summarized in table 4. Decisional needs were not consistently assessed or reported, with 10 studies (40.0%) not reporting any information on decisional needs of BCS experiencing side effects from OET. In the remaining 15 studies (62.5%), the most common categories of decisional needs were inadequate information (n=7 studies) and belief of benefits and risks (n=6 studies). One study described anxiety and uncertainty in BCS regarding their symptom experience, especially when physicians could not explain the exact etiology of their symptoms.26 Another study included data about BCS not having anyone to ask questions of and not understanding the duration, timing, or dose of their medication or having anyone available to answer questions.18
Discussion
In addition to providing a summary of the general nature of the studies that have been conducted on OET non-adherence in BCS who are experiencing side effects, there are 3 main findings resulting from this review. First, the review summarizes evidence on the relationship between the experience of side effects and OET non-adherence. Second, this review demonstrates the absence of decisional supports provided or available to BCS who are experiencing OET side effects. Third, this review demonstrates BCS have unmet decisional needs in their OET side effect-related decision-making processes. Each of these findings is discussed in detail below.
Relationship of non-adherence and side effects
The relationship between OET non-adherence and side effects underscores the importance of this clinical problem and provide evidence supporting the widespread notion that OET side effects are a major reason for non-adherence. Reported non-adherence rates are thought to be dependent on a range of parameters, including whether the patients are participating in a clinical trial, the period since initiating treatment, and methods used to assess adherence and medication use.27 It is likely that rates of non-adherence varied within these studies for similar reasons. Regardless of rates, non-adherence was primarily attributed to the experience of side effects. Within this literature, women who reported experiencing OET side effects were two to four times more likely to discontinue OET earlier than five years,5-9 and women who reported severe side effects were five times more likely to discontinue therapy earlier than five years.10 Though side effects caused women to switch to a different OET, switching does not prevent further side effects and many women subsequently discontinue even the second OET.11
Methods used to assess side effects of OET varied. Side effects were not assessed using comprehensive self-report measures, which interferes with understanding the true experience of the effect of these drugs. In addition, side effects were reported from overall OET, limiting our full understanding of side effects experienced by drug. Regardless, our review findings suggest that future research should be focused on improved understanding and elimination of non-adherence caused by side effects.
Absence of decisional supports
A second major finding of this review was the absence and inadequacy of available decisional supports for this population. The majority of current support was verbal direction from the provider occurring at the time of OET prescription. Details about existing support were limited, but when support was available, it was aimed mostly at the potential experience of side effects. Current support seemed to be lacking side effect management strategies or stressing the importance of remaining on a regimen even when experiencing side effects.
Even when BCS reported receiving support, they reported it was inadequate. BCS reported they were not given understandable OET-related information. The information they did receive was not sufficient, and they did not have the opportunity to ask questions. Limiting support to information only and not considering additional determinants of decision making such as unrealistic expectations, unclear values, unclear norms, or inadequate personal and external resources increases the potential for poor quality decisions.16
The absence of decisional support may be partially due to the lack of decisional support tools for this population. Decisional support tools often come in the form of a decision aid, which is an intervention that helps patients make specific and deliberative choices among options. Decision aids often provide information on treatment options and outcomes relevant to a person's health status, and they include methods to clarify patients' values.28 The Patient Decision Aids Research Group, affiliated with the Ottawa Hospital Research Institute, is an international research team that designs and tests decision aids and decisional support training programs for patients and health practitioners. The group manages a database of decision aids that can be uploaded and shared if they adhere to established guidelines, including that they (1) meet the definition of a decision aid, (2) are not more than 5 years old, (3) provide references to scientific evidence used, and (4) are publicly available.15 When the authors searched this database of decision aids that would support the OET decision-making process, only one tool was found. This decisional support tool is a decision aid for OET that focuses only on post-menopausal BCS making the initial decision to initiate therapy and does not take into consideration OET side effects or decision making as a process unfolding over time which can last 5-10 years.29 This further shows that there are inadequate resources for patients and providers to address the side effects and resulting impact on adherence of OET.
Unmet decisional needs
Importantly, this review showed that decisional needs are not systematically assessed in research or clinical practice. Assessment of decisional needs is important in decision making because it can identify what is important for the decision making, as well as what could be done better in the form of effective decisional support.15
A revealing finding from this review was the influence of beliefs about OET on adherence. BCS held complex beliefs about their OET, and for a number of BCS the decision to discontinue OET seemed to be the result of rational but misguided beliefs about their experience of side effects.30 Attempting to address their unmet decisional needs through seeking inaccurate information likely contributed to the formation of inaccurate beliefs about OET. This finding is important for adherence because it has been shown that BCS with negative or neutral beliefs about the value of OET were more likely to discontinue it.7 BCS report having unmet needs regarding information they receive, and they report seeking additional information from sources other than their provider. BCS report having unmet needs regarding information they receive, and they report seeking additional information from sources other than their provider. Though BCS turn to alternative sources for OET-related information, these sources may not provide adequate benefit due to uneven quality, conflicting claims, redundancy, and difficulties associated with assessing information accuracy and applicability.31
Limitations
Review findings should be interpreted in light of some limitations. First, information on needs and support had to be extracted from methods and results sections. Thus, our findings may actually under-represent BCS's supports and needs, suggesting that a more detailed and purposeful study of supports and needs is warranted. A logical next step for research would be to conduct a detailed, basic, descriptive study of BCS's decision-making processes and the unfolding of their decisional needs and supports over time. Second, the literature search was limited to English language articles and a single comprehensive search engine. Search limitations could have limited the search results and potentially omitted additional findings published in other languages or identified in less popular journals not indexed within PubMed or CINAHL.
Conclusions
Overall, the prevalence of side effects was quite high and was cited as the major reason for discontinuing OET. Our study confirms that non-adherence to OET due to the experience of side effects remains an importance issue, primarily because BCS experiencing OET side effects have unmet decisional needs and lack adequate decisional supports.
This review indicates that more decisional support for BCS experiencing side effects related to OET may be needed. Although we know that side effects contribute to BCS's decisions to stop OET, we do not understand the process through which that occurs. In addition, although we know that BCS state they receive insufficient information about side effects from providers and seek out additional information, we do not fully understand that process or how it may relate to decision making. Future research is needed to further define the concepts of decisional needs and decisional supports for BCS experiencing side effects from OET in order to develop patient-centered materials to improve outcomes of OET therapy. Narrative accounts by BCS who are experiencing OET side effects will provide foundational descriptive information needed to generate interventions to improve quality decision making, such as a decision aid. In order to address the gap in currently available decision aids, next steps should include qualitative descriptive research to generate a full understanding of the decision-making process in BCS who experience OET side effects.
Implications for practice
This review generates some insights for providers who treat BCS with OETs, particularly when they are assessing OET adherence and side effects. The decision to take OET is not a single event decision, but a complex social process that occurs over time as a series of one time daily decisions or twice daily decisions over the course of up to 10 years. This decision making is further complicated for BCS who experience side effects. Categories of side effects, adherence, decisional support, and decisional needs are all associated with OET decision making, and each of these categories is associated with specific clinical implications as discussed below.
At some point during OET treatment, a large proportion of BCS most likely experience some type of side effect. 5-7, 10-12, 17-20, 23-26, 30, 32-35 Inadequately managed side effects potentially increase non-adherence, leading to an increased risk of breast cancer recurrence.2,3 Current methods to assess side effects are inconsistent and unstandardized across the research literature.36 Existing literature suggests providers are failing to document the assessment of side effects. Furthermore, this review indicates that little is known about how information regarding side effects is communicated. Clinician recorded side effects tend to emphasize serious, life-threatening adverse events rather than patient-reported issues affecting quality of life. Information communicated to women by providers may not fully encompass the true side effect burden that may result from OET. Poor or inadequate communication fuels lack of understanding which can further negatively impact clinicians' abilities to support BCS in the management of their side effects and poor quality decisions made by BCS regarding their OET. We recommend that provider assessments include patient report of the experience of side effects from OET at every clinical visit as well as an assessment of adherence.
Decisional support for BCS can be provided in several different ways. Decisional support from providers may include health messages about the importance of continued OET or include a decisional support tool that addresses the problem (side effects from OET), alternatives, benefits, and risks related to deciding to take or not to take prescribed therapies. By providing decisional support to BCS using these methods, unmet decisional needs may be minimized, leading to a quality decision. Results of this review suggest the lack of decisional support for BCS lead to unmet decisional needs and provide a basis to guide health provider encounters with BCS taking OET.
According to the Ottawa Decision Support Framework, the primary driver of whether individuals are able to make quality decisions is whether their decisional needs are understood and supported.15 BCS who are inadequately informed about OET side effects or the importance of adherence are likely to have unmet decisional needs. By identifying unmet decisional needs, health providers can then be guided towards the types of patient centered OET health information BCS need in order to have adequate support. Providers can determine unmet decisional needs and tailor decisional support provided to BCS during patient encounters resulting in quality decisions that lead to side effect management ultimately resulting in improved adherence to OET.
Acknowledgments
Sources of Support: This project was supported by the grant number R36HS024241 from the Agency for Healthcare Research and Quality (Milata PI), grant number 2T32 NR007066 (Rawl, PI) from the National Institute of Nursing Research, a research doctorate scholarship from the Oncology Nursing Society Foundation, and research incentive funding from the Indiana University School of Nursing. The content is solely the responsibility of the author and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or of the National Institute of Nursing Research.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Society AC. Cancer Facts and Figures, 2015. Atlanta. 2015 [Google Scholar]
- 2.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1-2):1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 3.Luschin G, Habersack M. Oral Information About Side Effects of Endocrine Therapy for Early Breast Cancer Patients at Initial Consultation and First Follow-Up Visit: An Online Survey. Health Commun. 2013 doi: 10.1080/10410236.2012.743096. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed 05/24/2015];Wolters Kluwer Health. http://www.uptodate.com. Available at: http://www.uptodate.com.
- 5.Mao JJ, Chung A, Benton A, et al. Online discussion of drug side effects and discontinuation among breast cancer survivors. Pharmacoepidemiology and drug safety. 2013;22(3):256–262. doi: 10.1002/pds.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles EJA, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. Journal of Oncology Practice. 2012;8(6):e149–e157. doi: 10.1200/JOP.2012.000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient education and counseling. 2005;59(1):97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Medical care. 2007;45(5):431–439. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 10.Bell RJ, Fradkin P, Schwarz M, Davis SR. Understanding discontinuation of oral adjuvant endocrine therapy by women with hormone receptor–positive invasive breast cancer nearly 4 years from diagnosis. Menopause. 2013;20(1):15–21. doi: 10.1097/gme.0b013e3182610cab. [DOI] [PubMed] [Google Scholar]
- 11.Boonstra A, van Zadelhoff J, Timmer-Bonte A, Ottevanger PB, Beurskens CH, van Laarhoven HW. Arthralgia during aromatase inhibitor treatment in early breast cancer patients: prevalence, impact, and recognition by healthcare providers. Cancer nursing. 2013;36(1):52–59. doi: 10.1097/NCC.0b013e31824a7e18. [DOI] [PubMed] [Google Scholar]
- 12.Chim K, Xie SX, Stricker CT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC cancer. 2013;13(1):401. doi: 10.1186/1471-2407-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. Journal of Clinical Oncology. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schover LR, Baum GP, Fuson LA, Brewster A, Melhem-Bertrandt A. Sexual Problems During the First 2 Years of Adjuvant Treatment with Aromatase Inhibitors. The journal of sexual medicine. 2014 doi: 10.1111/jsm.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor A. Ottawa Decision Support Framework to address decisional conflict. 2006;2012 doi: 10.1177/0272989X06290492. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33(3):267–279. doi: 10.1016/s0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 17.Cluze C, Rey D, Huiart L, et al. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Annals of oncology. 2012;23(4):882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 18.Harrow A, Dryden R, McCowan C, et al. A hard pill to swallow: a qualitative study of women's experiences of adjuvant endocrine therapy for breast cancer. BMJ open. 2014;4(6):e005285. doi: 10.1136/bmjopen-2014-005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp A, Preen DB, Saunders C, et al. Early discontinuation of endocrine therapy for breast cancer: who is at risk in clinical practice? SpringerPlus. 2014;3(1):282. doi: 10.1186/2193-1801-3-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyvernitakis I, Ziller V, Hars O, Bauer M, Kalder M, Hadji P. Prevalence of menopausal symptoms and their influence on adherence in women with breast cancer. Climacteric. 2013(0):1–8. doi: 10.3109/13697137.2013.819327. [DOI] [PubMed] [Google Scholar]
- 21.Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast cancer research and treatment. 2014;145(2):525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]
- 22.Wouters H, van Geffen EC, Baas-Thijssen MC, et al. Disentangling breast cancer patients' perceptions and experiences with regard to endocrine therapy: Nature and relevance for non-adherence. The Breast. 2013;22(5):661–666. doi: 10.1016/j.breast.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. Journal of Clinical Oncology. 2001;19(2):322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 24.Kirk MC, Hudis CA. Insight into barriers against optimal adherence to oral hormonal therapy in women with breast cancer. Clin Breast Cancer. 2008;8(2):155–161. doi: 10.3816/CBC.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 25.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini I, Sarradon-Eck A, Ben Soussan P, et al. Women's perceptions and experience of adjuvant tamoxifen therapy account for their adherence: breast cancer patients' point of view. Psycho-Oncology. 2010;19(5):472–479. doi: 10.1002/pon.1593. [DOI] [PubMed] [Google Scholar]
- 27.Hadji P, Blettner M, Harbeck N, et al. The Patient's Anastrozole Compliance to Therapy (PACT) Program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Annals of oncology. 2013 doi: 10.1093/annonc/mds653. mds653. [DOI] [PubMed] [Google Scholar]
- 28.Giguere A, Legare F, Grad R, et al. Developing and user-testing Decision boxes to facilitate shared decision making in primary care-a study protocol. BMC medical informatics and decision making. 2011;11(1):17. doi: 10.1186/1472-6947-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ASCO. DECISION AID TOOL. Adjuvant Endocrine Therapy for Hormone Receptor-Positive Breast Cancer. 2010 [Google Scholar]
- 30.Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59(1):97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Rahm AK, Hawkins RP, Dearing JW, et al. Implementing an evidence-based breast cancer support and communication tool to newly diagnosed patients as standard care in two institutions. Transl Behav Med. 2015;5(2):198–206. doi: 10.1007/s13142-015-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. European Journal of Cancer. 2006;42(14):2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Bramwell V, Pritchard K, Tu D, et al. A randomized placebo-controlled study of tamoxifen after adjuvant chemotherapy in premenopausal women with early breast cancer (National Cancer Institute of Canada—Clinical Trials Group Trial, MA. 12) Annals of Oncology. 2010;21(2):283–290. doi: 10.1093/annonc/mdp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45(5):431–439. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 36.Simon R, Latreille J, Matte C, Desjardins P, Bergeron E. Adherence to adjuvant endocrine therapy in estrogen receptor–positive breast cancer patients with regular follow-up. Canadian Journal of Surgery. 2014;57(1):26. doi: 10.1503/cjs.006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, web of science, and Google scholar: strengths and weaknesses. The FASEB journal. 2008;22(2):338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 38.Joseph P Healey Library. Ask a Librarian. Universiy of Massachusetts Boston: [Retrieved 4/15/2016]. http://umb.libanswers.com/a.php?qid=159963. [Google Scholar]