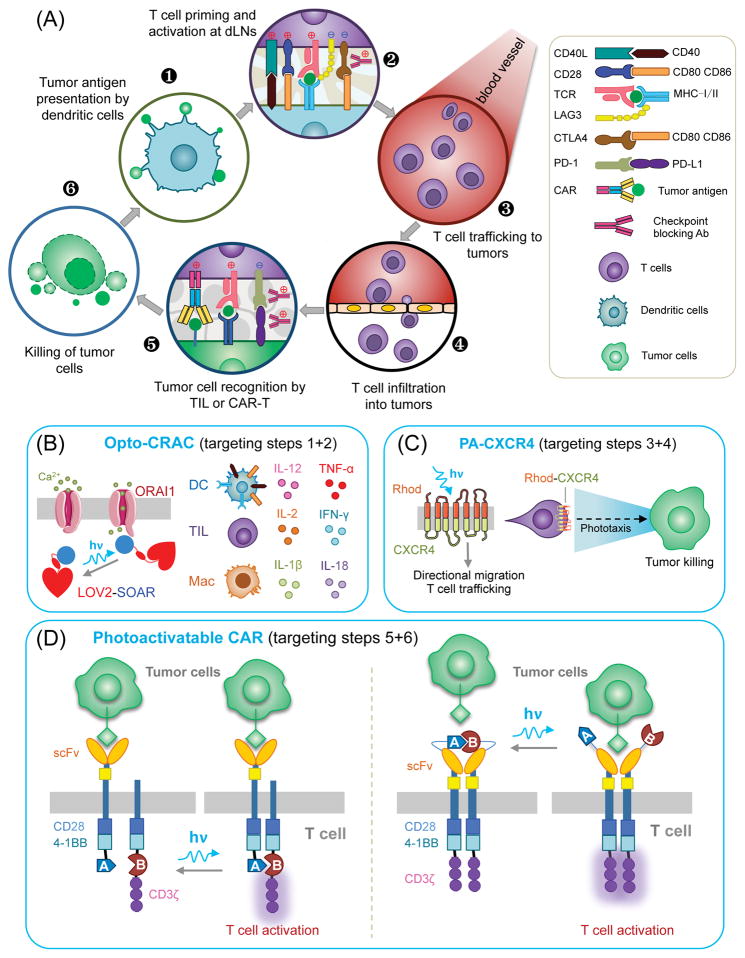
Figure 1, Key Figure. The cancer-immunity cycle and opportunities for optogenetic interventions to improve cancer immunotherapies.
(A) A typical anti-tumor immune response involves the following major steps: (1) Tumor antigens are released and captured by dendritic cells (DCs) for processing; (2) DCs present processed antigens to T cells to prime and activate TCR signaling against tumor-specific antigens in tumor draining lymph nodes (dLNs). T-cell antitumor activity is balanced by coordinated actions of both stimulatory (red-cross) and inhibitory (blue-cross) signals. (3) Activated effector T cells travel through blood vessels and (4) infiltrate into tumor sites by crossing endothelial barrier. (5) Tumor-infiltrating T cells (TILs) recognize cancer cells through the interaction between TCR and cognate antigens bound to MHC-I on tumor cells. Engineered therapeutic T cells expressing chimeric antigen receptor (CAR-T) are also used in the clinic to boost tumor killing. Immunomodulatory monoclonal antibodies can overcome local immunosuppression by blocking suppressive PD-1 receptors and (6) eventually initiate the tumor cell killing machinery. CTLA-4, cytotoxic T-lymphocyte antigen-4; PD-1, programmed cell-death 1; LAG-3, lymphocyte activation gene-3; 4-1BB, tumor necrosis factor receptor superfamily member 9.
(B) Opto-CRAC channels engineered into DCs, TILs, or macrophages (Mac) to photo-manipulate calcium-dependent events in the immune system (e.g., antigen presentation and cytokine production). LOV, light-oxygen-voltage domain from phototropin 1; SOAR/CAD, the STIM-Orai-activating domain/the CRAC activation domain.
(C) Photoactivatable rhodopsin (Rhod)-CXCR4 chimeric chemokine receptor enables T cells trafficking and phototaxis to boost tumor killing.
(D) Proposed strategies for photomanipulating engineered CAR-T cells via (left) heterodimerization of co-stimulation molecules or (right) conformational switch in the extracellular single-chain variant fragment (scFv).
Blue arrow, photoexcitation with blue light; Black arrow, reversal to the dark state.