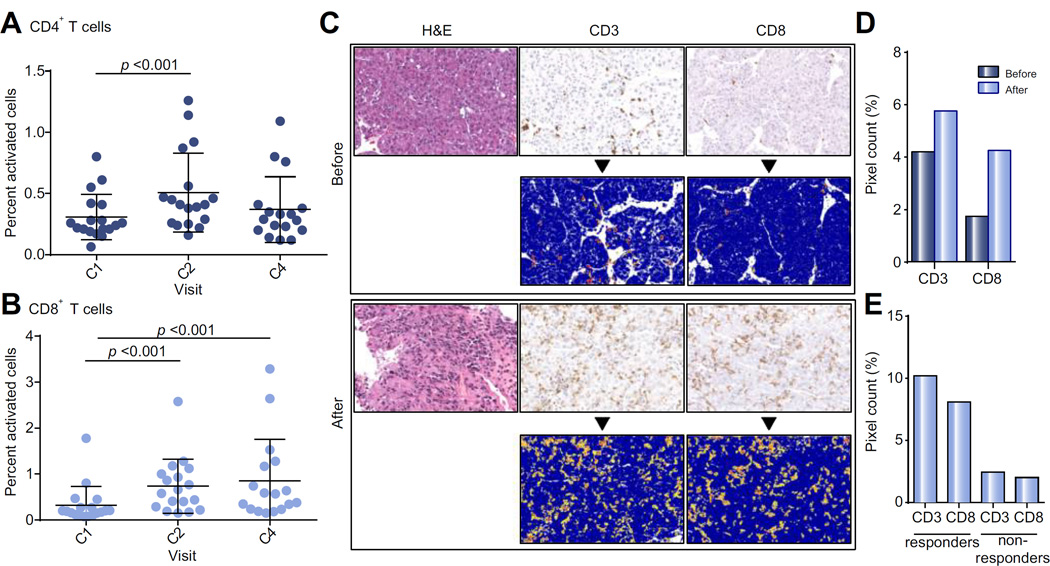
Fig. 3. Correlative studies.
(A) Percent of live activated CD4+ T cells in peripheral blood of patients during first 4 cycles of tremelimumab. Wilcoxon signed rank test was used to compare T cell subsets at baseline with samples obtained after 4 and 12 weeks. (B) Percent of live activated CD8+ T cells in peripheral blood of patients during first 4 cycles of tremelimumab. Wilcoxon signed rank test was used to compare T cell subsets at baseline with samples obtained after 4 and 12 weeks. (C) Representative tumor biopsy at baseline and post 2 doses of tremelimumab showing marked intratumoral CD3+/CD8+ T cell infiltration (bottom rows: positive pixel count overlay). (D) Quantitative assessment of immunohistochemical staining showing average CD3 and CD8 immune cell tumor infiltration before (N = 6) and after 2 doses of tremelimumab (N = 12). (E) Quantitative assessment of immunohistochemical staining showing average CD3 and CD8 immune cell tumor infiltration after 2 doses of tremelimumab for patients divided into responders (defined as stable/partial response of at least 4 months, N = 5) vs. non-responders (defined as PD/partial response of under 4 months, N = 4).