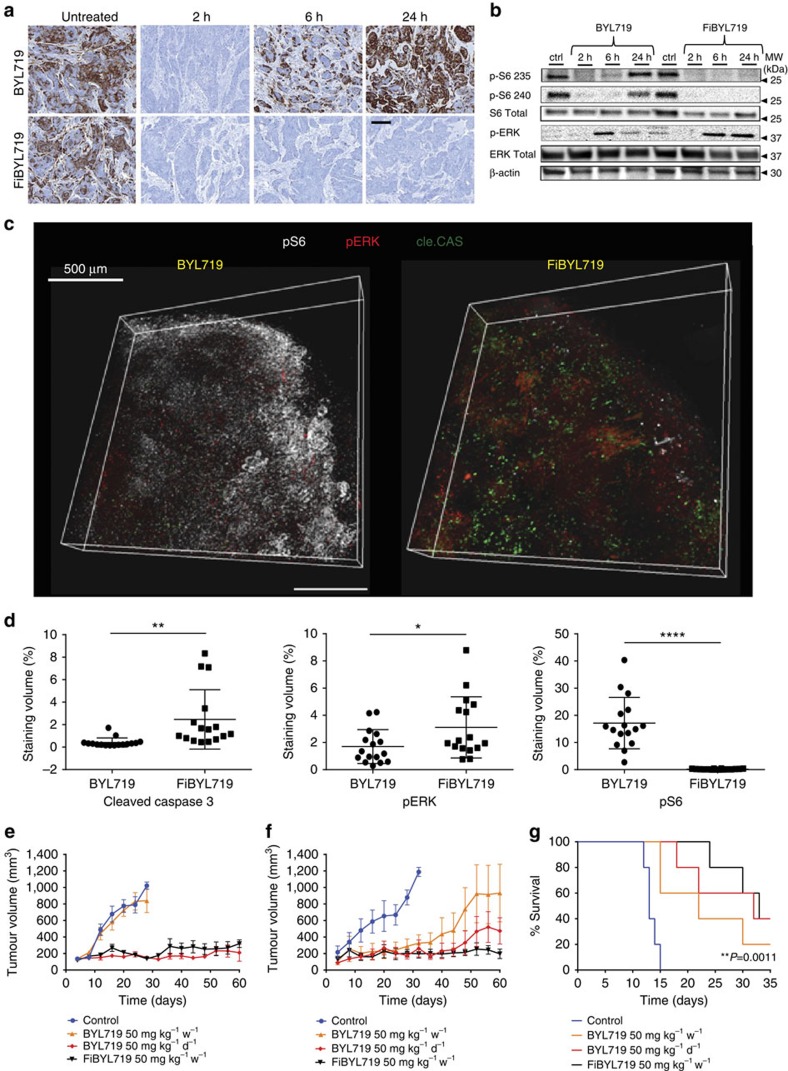
Figure 2. Anti-tumour efficacy of free BYL719 and nanoparticle-encapsulated FiBYL719 in pre-clinical HNSCC models.
(a) Representative images of immunohistochemistry staining for pS6 at different time points following treatment with 25 mg kg−1 BYL719 or 25 mg kg−1 FiBYL719 in Cal-33 xenografts (n=3). Scale bar, 50 μm. (b) Western blot of pS6 and pERK in Cal-33 xenograft tissues following treatment with 25 mg kg−1 BYL719 or 25 mg kg−1 FiBYL719, n=3. (c) 3-D reconstruction of a stained Cal-33 xenograft section 24 h after treatment with either 50 mg kg−1 BYL719 or 25 mg kg−1 FiBYL719. pS6 (white), pERK (red) and cleaved caspase 3 (green). Scale bar, 500 μm. (d) Box plots comparing the volume of positive staining (% of total tissue volume) of tissues shown in c, (n=2). (e) Tumour growth curves of Cal-33 xenografts treated with oral administration of either 50 or 7 mg kg−1 BYL719 daily, or i.v. injection of 25 mg kg−1 FiBYL719 bi-weekly (n=10). (f) Tumour growth curves of H22 patient-derived xenografts treated with oral administration of either 50 or 7 mg kg−1 BYL719 daily, or bi-weekly i.v. injections of 25 mg kg−1 FiBYL719 (n=10). (g) Survival curve of mice engrafted with orthotopic tongue cal-33 xenografts treated with oral administration of either 50 or 7 mg kg−1 BYL719 daily or i.v. injections of 25 mg kg−1 FiBYL719 bi-weekly (n=5). In d–f, error bars indicate mean±s.e.m. *P<0.05, **P<0.01, ****P <0.0001; by one-way ANOVA with post hoc Tukey test. In g, P-value was calculated by using the log-rank test.