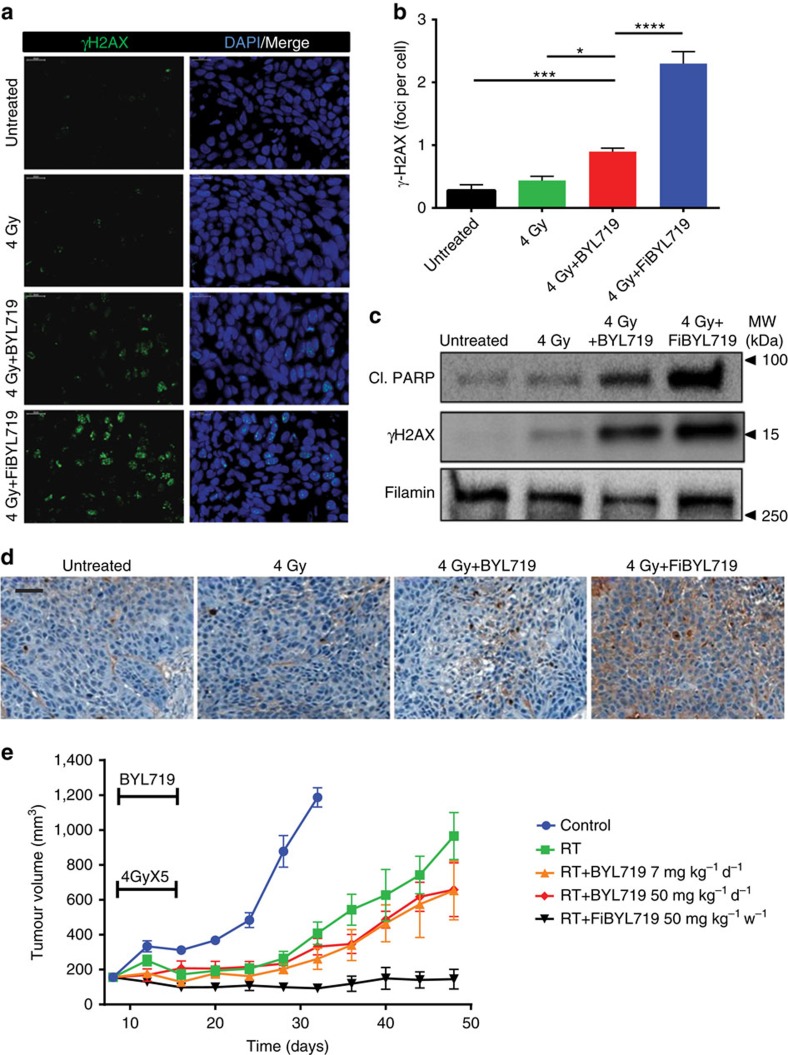
Figure 3. Radiosensitization effects of pre-clinical HNSCC models by free and nanoparticle-encapsulated BYL719.
(a) Representative images of immunofluorescence staining for nuclear γH2AX foci (green) and DAPI (blue) in H22 patient-derived xenografts 24 h post treatment with RT (4 Gy) or RT followed by 50 mg kg−1 BYL719 or 25 mg kg−1 FiBYL719. Scale bar, 20 μm. (b) Quantification of γH2AX staining (foci per cell) presented in a (n=3). (c) Western blot of γH2AX and cleaved PARP in H22 patient-derived xenografts 24 h post treatment with RT (4 Gy), RT and 50 mg kg−1 BYL719, or 25 mg kg−1 FiBYL719 (n=3). (d) Representative images of immunohistochemical staining for TUNEL in H22 patient-derived xenografts 24 h post treatment with 4 Gy RT, 4 Gy RT plus 50 mg kg−1 BYL719, or 4 Gy RT plus 25 mg kg−1 FiBYL719. Scale bar, 50 μm. (e) Tumour growth curves of H22 patient-derived xenografts treated for 5 days with daily oral administration of either 50 or 7 mg kg−1 BYL719 daily, or with i.v. injections of 25 mg kg−1 FiBYL719 administered bi-weekly, combined with fractionated RT of 4 Gy × 5 doses on days 1–5 (n=10). In b,e, error bars indicate mean±s.e.m. *P<0.05 ***P<0.001, ****P <0.0001; by one-way ANOVA with post hoc Tukey test.