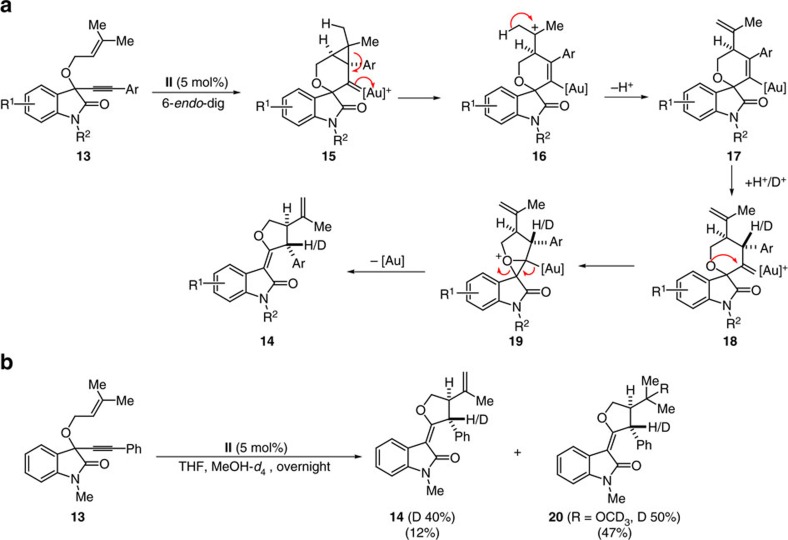
Figure 5. Mechanistic proposal for the formation of df-oxindole 14.
(a) The proposed mechanism of the formation of df-oxindole 14 involves the gold(I)-mediated 6-endo-dig cycloisomerization of 1,6-enyne to afford 15, followed by subsequent deprotonation, protonation, formation of a strained tetracyclic spiro-oxenium intermediate (19) and its rearrangement to afford 14. (b) In the presence of d-MeOH (as nucleophile as well as deuterium source), df-oxindole 14 and d-MeOH adduct 20 of df-oxindole was obtained in 14% yield with 40% deuteration at benzylic position and 47% yield with 50% deuteration at benzylic position, respectively. MeOH-d4, deuterated methanol.