Table 1. Experimental and calculated polarization of PTM ferroelectric crystals.
| Compound |
Polarization (μC cm−2) |
Direction component | Local dipole moment of proton transfer | |||||
|---|---|---|---|---|---|---|---|---|
|
Experimental |
Theoretical (Berry) |
Point charge model |
(x, y, z) | |μ| (10−30 C m) | ||||
| |Pexp| | (Px, Py, Pz)exp | |Pcal| | (Px, Py, Pz)cal | |Pion| | (Px, Py, Pz)ion | |||
| 1. CRCA | 30 | (0, 0, 30) | 29.4 | (0, 0, 29.4) | 5.5 | (0, 0, 5.5) | (a, b, c) | 4.4, 5.0 |
| 2. PhMDA | 9 | (0, 0, 9) | 9.0 | (0, 0, 9.0) | 1.8 | (0, 0, 1.8) | (a, b, c) | 5.3 |
| 3. HPLN | 5.6* | — | 5.2 | (−5.2, 0, −0.1) | 2.5 | (0, 0, 0) | (a, b, c*) | 5.9, 6.0 |
| 4.5 | 4.2 | ⊥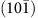
|
||||||
| 4. CBDC | 13.2 | (8.6, 0, −10.0)† | 15.1 | (11.7, 0, −9.6) | 6.7 | (6.3, 0, −2.2) | (a, b, c*) | 5.5 |
| 5. MBI | 7.4* | — | 7.1 | (−0.2, 0, −7.1) | 2.5 | (−0.2, 0 −2.5) | (a, b, c*) | 6.7, 5.7, 6.7, 6.1 |
| 5.2 | 5.0 | ||[101] | ||||||
| 6. DC-MBI | 10 | (0, 0, −10) | 10.0 | (0, 0, −10.0) | 3.8 | (0, 0, 3.8) | (a, b, c) | 8.3 |
| 7. ALAA | 3.6 | (0, 0, 3.6) | 4.2 | (0, 0, 4.2) | 0.6 | (0, 0, 0.6) | (a, b, c) | 7.5 |
ALAA, 3-anilinoacrolein anil; CBDC, cyclobutene-1,2-dicarboxylic acid; CRCA, croconic acid; DC-MBI, 5,6-dichloro-2-methylbenzimidazole; HPLN, 3-hydroxyphenalenone; PhMDA, 2-phenylmalondialdehyde; PTM, prototropy.
*The direction of the applied field exhibits a certain inclination angle from the polarization vector. The total amplitude of experimental polarization |Pexp| was derived in consideration of this (theoretical) angle.
†Because the sign of each direction component of Pexp could not be identified by the P−E hysteresis experiments alone, it is assumed to be the same as that of Pcal.
