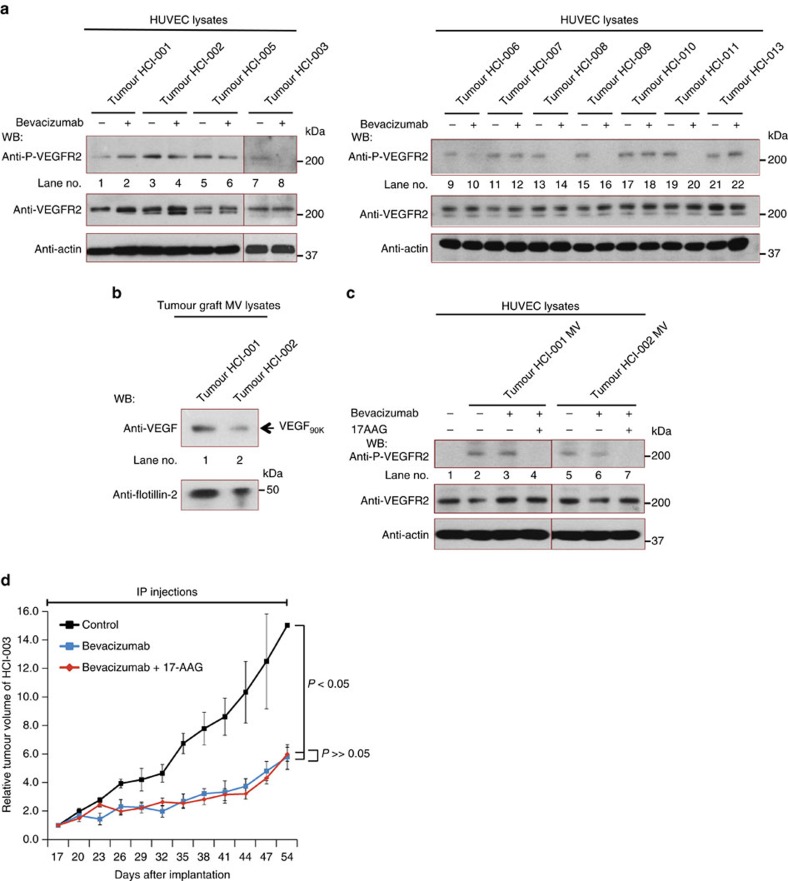
Figure 7. The role of MVs in Bevacizumab insensitivity extends to PDXs.
(a) Lysates of serum-deprived HUVECs treated with conditioned medium (CM; 20 μg ml−1 total protein) from cell cultures of PDX samples HCI-001-003 and HCI-005 (Left) or HCI-006-011 and HCI-013 (Right), described in Table 1, were supplemented without or with 0.5 μg ml−1 Bevacizumab, as indicated, for 15 min. HUVEC lysates were immunoblotted with antibodies that recognize phosphorylated VEGFR2, total VEGFR2 or actin. (b) MVs from cultures of cells established from PDX samples HCI-001 and HCI-002 were isolated, lysed and immunoblotted with antibodies against pan VEGF or the MV marker flotillin-2. (c) Serum-deprived HUVECs were incubated with serum-free medium alone (control; lane 1), or with serum-free medium containing the indicated combinations of MVs from PDX samples HCI-001 or HCI-002 (5 μg ml−1 of MV protein), 0.5 μg ml−1 Bevacizumab (lanes 3 and 6) and 10 μM 17AAG (lanes 4 and 7), for 15 min and then lysed. Cell extracts were immunoblotted with antibodies that recognize phosphorylated VEGFR2, total VEGFR2 or actin. (d) Plots showing relative mean tumour volumes (mm3) of tumour graft HCI-003 in NOD/SCID mice that were untreated (vehicle control only), or treated with either Bevacizumab or Bevacizumab plus 17AAG. The differences between the tumour volumes for the Bevacizumab treatment group versus the vehicle only (control) group were statistically significant (P<0.05).