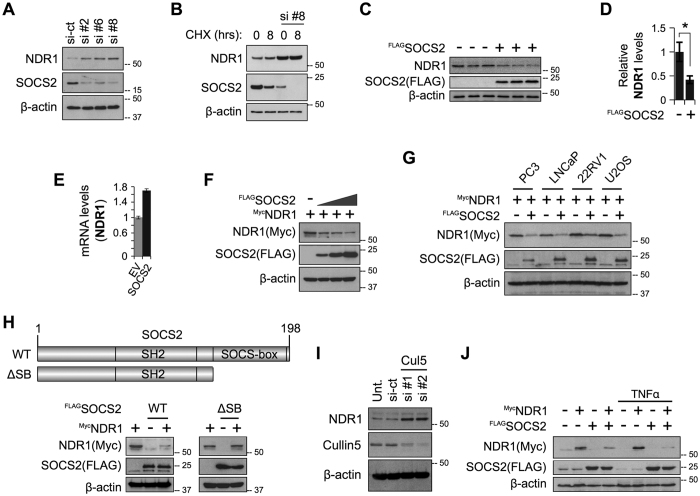
Figure 2. SOCS2 destabilizes NDR1.
(A) Blots prepared in Fig. 1A were re-probed for NDR1. (B) MEFs were transfected with either a NT siRNA or si-SOCS2 #8 and 24 hrs later treated with cycloheximide for additional 8 hours. Lysates were propped by IB. (C) MEFs were transfected with FLAG-SOCS2 plasmid. Lysates from three independent experiments were probed by IB. (D) Pixel densitometry values obtained using ImageJ and normalized against β-actin are shown as a bar graph representing mean ± SEM, *P value < 0.05. (E) QPCR analysis of NDR1 gene using total RNAs extracted from MEFs transfected with FLAG-SOCS2 or an empty vector (EV). 18S rRNA was used as internal control. (F) MEFs were transfected with a constant amount of a plasmid encoding Myc-NDR1 and an increasing amount of FLAG-SOCS2, while keeping the total amount of DNA equal using EV. IB was done using indicated antibodies. (G) Plasmid for Myc-NDR1 were co-transfected with either EV or FLAG-SOCS2 in a panel of different cell lines and lysates were probed by IB. (H) MEFs were transfected with Myc-NDR1 in combination with either FLAG-SOCS2 or FLAG-SOCS2-ΔSB plasmids as shown. Lysates were probed by IB. (I) RNAi mediated depletion of Cullin5 in MEFs and IB for NDR1. (J) Plasmid for Myc-NDR1 were co-transfected with either EV or FLAG-SOCS2 and treated with TNFα (10 ng/ml for 1 hour) as shown. Lysates were probed by IB. Full-length blots of 2C (SOCS2) and 2C (β-actin) blots are presented in Supplementary Figure S4.