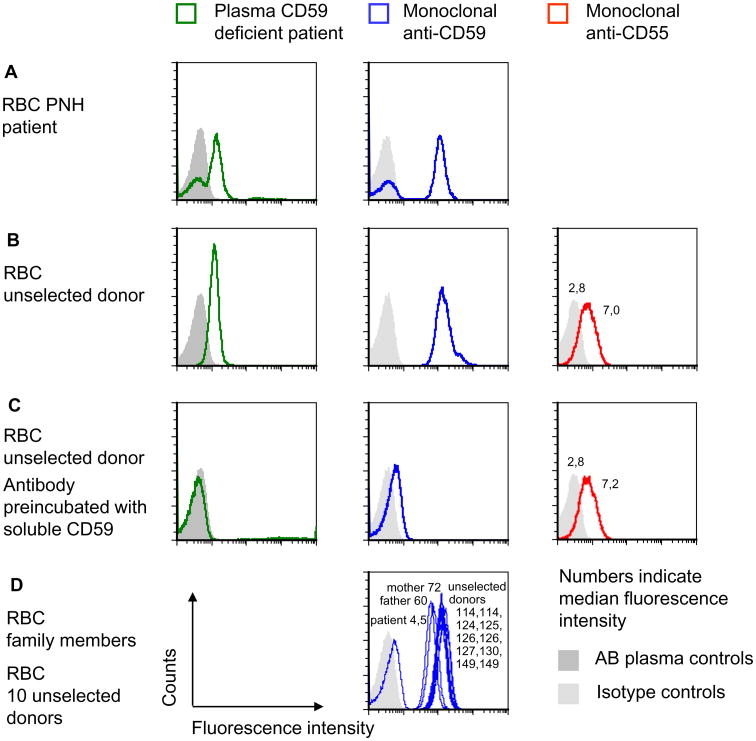
Fig. 1.
Identification of anti-CD59 alloantibody by flow cytometry. (A) The fractions of CD59-positive and -negative RBCs of patients with PNH were determined by flow cytometry using plasma of the patient with CD59 deficiency or monoclonal anti-CD59. Controls received either isotype control or pooled AB plasma instead of the patient plasma. The data shown are representative of three independent experiments with similar results. (B) RBCs of an unselected donor were incubated with plasma of the CD59-deficient patient, with FITC-labeled anti-CD59 or PE-labeled anti-CD55, respectively, and analyzed by flow cytometry. The data shown are representative for two independent experiments with similar results. (C) The same assays were performed as in B except that recombinant CD59 was added to the antiserum or to the plasma of the patient. The amount of anti-CD55 used was lower than the amount of anti-CD59 to demonstrate the complete absence of a blocking effect of soluble CD59. The data shown are representative of two independent experiments with similar results. (D) The RBCs of the mother and the father of the CD59-deficient patient were tested by flow cytometry using FITC-labeled monoclonal anti-CD59. Ten unselected donors were analyzed as controls; each sample was accompanied by an isotype control, and the filled light gray area indicates the isotype controls of all experiments depicted in D. The data shown are representative of two experiments with similar results.