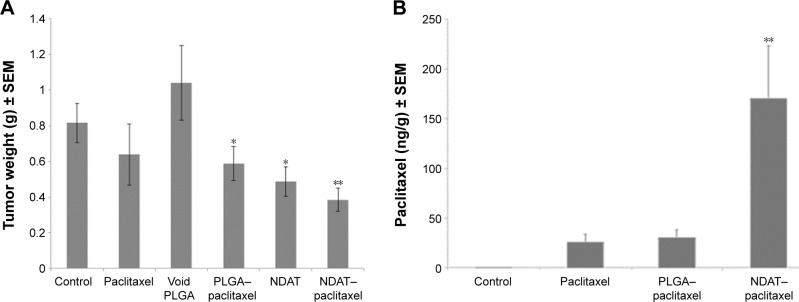
Figure 3.
Effect on tumors of pancreatic SUIT2-luc cancer cell xenografts of daily s.c. administration of control (PBS), paclitaxel, void PLGA, PLGA–paclitaxel (paclitaxel encapsulated in PLGA nanoparticles, without tetrac), low-dose NDAT (0.3 mg/kg b.w., with empty payload compartment), and NDAT (0.3 mg/kg b.w.)–paclitaxel (0.3 kg/mg b.w.).
Notes: (A) Tumor weights: weights were measured of harvested grafts at animal sacrifice. Significant tumor weight reduction was achieved with PLGA–paclitaxel, NDAT, and NDAT–paclitaxel. n=8–9 mice per group, * P<0.01 vs void PLGA, ** P<0.001 vs void PLGA or NDAT–paclitaxel. (B) Paclitaxel uptake by pancreatic cancer tumors in response to administration of control (PBS), paclitaxel, PLGA–paclitaxel, and NDAT–paclitaxel measured with LC-MS/MS. Tumor content of paclitaxel in grafts exposed to NDAT–paclitaxel was 5.2-fold that achieved with paclitaxel treatment alone or PLGA–paclitaxel. ** P<0.001 vs paclitaxel and PLGA–paclitaxel.
Abbreviations: b.w., body weight; LC-MS/MS, liquid chromatography-mass spectroscopy/mass spectroscopy; NDAT, nano-diamino-tetrac; PBS, phosphate-buffered saline; PLGA, poly(lactic-co-glycolic acid); s.c., subcutaneous; SEM, standard error of the mean; tetrac, tetraiodothyroacetic acid.