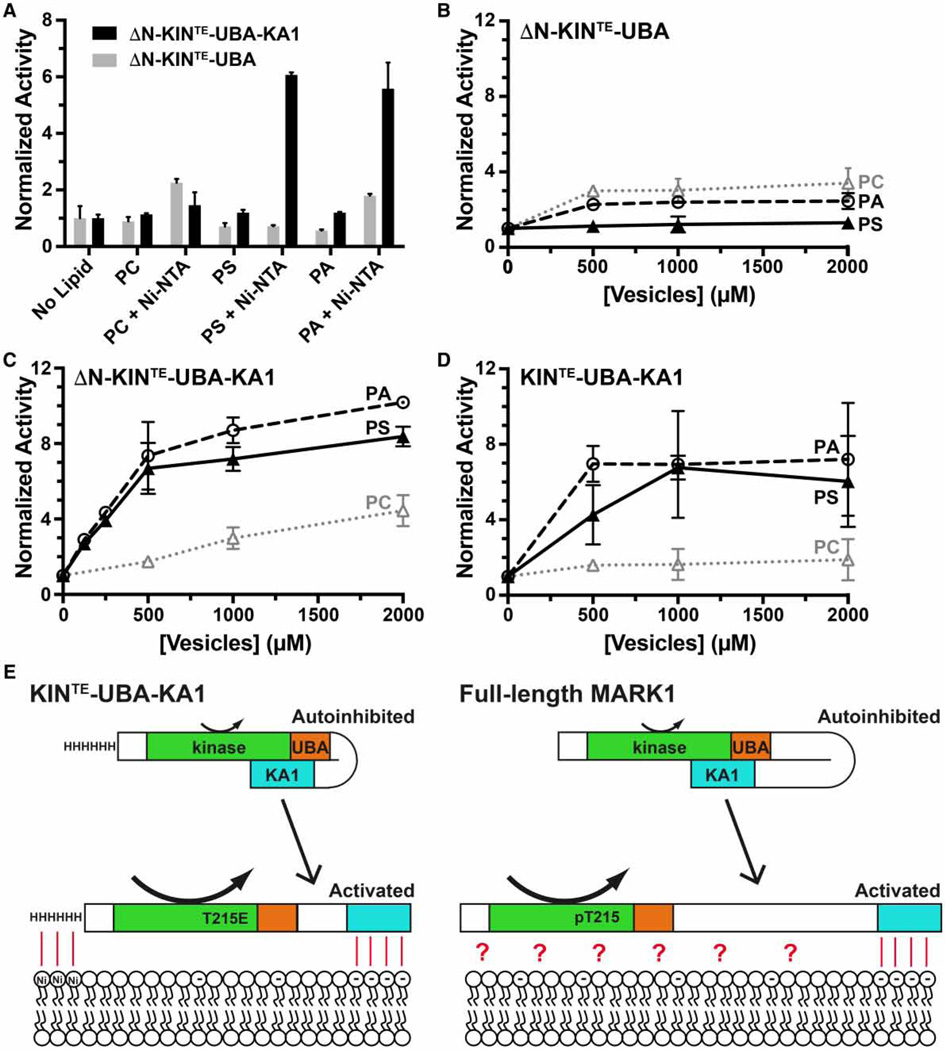
Figure 6. Lipid activation of ‘mini’ MARK1.
(A) Kinase activities of the ΔN-KINTE-UBA-KA1 ‘mini’ MARK1 and a variant lacking the KA1 domain (ΔN-KINTE-UBA) were assayed in triplicate following the addition of 200 µM DOPC, in vesicles into which various combinations of other phospholipids had been incorporated. Values are plotted as mean ± standard deviation, normalized to activity in the absence of added lipid. The data show that vesicles containing PS or PA robustly activated ‘mini’ MARK1, but only when they also contained 5% (mole/mole) DOGS-Ni-NTA, which binds the hexahistidine tag on both constructs — left intact for these experiments. PS or PA was included at 20% (mole/mole) when present as the only addition, or 15% when added alongside DOGS-Ni-NTA. (B) The kinase activity of ΔN-KINTE-UBA (lacking the KA1 domain) was unaffected by the addition of vesicles containing 5% (mole/mole) DOGS-Ni-NTA plus 95% PC alone (dotted gray line), 80% PC plus 15% PA (dashed black line), or 80% PC plus 15% PS (solid black line). Vesicles were titrated up to 2 mM total added lipid. (C) By contrast, histidine-tagged ΔN-KINTE-UBA-KA1 ‘mini’ MARK1 was robustly activated by the vesicles containing DOGS-Ni-NTA plus PA or PS, as was KINTE-UBA-KA1 (D). We present a hypothetical model in (E) where MARK1 can be activated by the presence of anionic phospholipids (and their binding to the KA1 domain), but only when another entity contributes to membrane recruitment. This role is assumed by the hexahistidine tag binding to DOGS-Ni-NTA in our in vitro studies, but might be provided by septins in yeast orthologs [12, 43], and/or other components (denoted by the red question mark) in MARK1 — as summarized in the text. Curved arrows indicate low kinase activity for the autoinhibited state, and elevated kinase activity in the membrane-bound state.