SUMMARY
Medulloblastoma is the most common malignant brain tumor in children. Aberrant activation of the hedgehog signaling pathway is strongly implicated in the development of some cases of medulloblastoma. A 26-year-old man with metastatic medulloblastoma that was refractory to multiple therapies was treated with a novel hedgehog pathway inhibitor, GDC-0449; treatment resulted in rapid (although transient) regression of the tumor and reduction of symptoms. Molecular analyses of tumor specimens obtained before treatment suggested that there was activation of the hedgehog pathway, with loss of heterozygosity and somatic mutation of the gene encoding patched homologue 1 (PTCH1), a key negative regulator of hedgehog signaling.
Medulloblastoma is a malignant tumor of the cerebellum. The median age at diagnosis is 5 years, with the age range extending into young adulthood. Primary management consists of surgical resection followed by radiation therapy and chemotherapy. Current therapies have serious short-term and long-term adverse effects, including postoperative mutism, neurocognitive deficits, endocrinopathies, sterility, and the risk of secondary high-grade glioma or meningioma.1 Patients with recurrent disease after primary therapy have a particularly poor prognosis, with a median survival of less than 6 months; the 2-year survival rate among these patients is approximately 9%.2
The hedgehog pathway is an essential embryonic signaling cascade that regulates stem-cell and progenitor-cell differentiation in multiple developmental processes.3 Smoothened homologue (SMO) is a transmembrane protein that activates the downstream hedgehog signaling pathway. PTCH1 is an inhibitory cell-surface receptor that constitutively suppresses activation of the hedgehog pathway by inhibiting SMO. Hedgehog ligands bind to and inactivate PTCH1, derepressing SMO and promoting pathway activation.
Medulloblastoma is thought to arise from stem cells or early progenitor cells in the cerebellum.4 A critical developmental process in cerebellar maturation involves expansion, migration, and differentiation of immature precursor cells from the external granule-cell layer to form the internal granule-cell layer.5 This process is spatially and temporally regulated by activation of the hedgehog pathway in granule-cell precursors.6
Both preclinical and clinical observations suggest that aberrant ligand-independent constitutive activation of the hedgehog signaling pathway in granule-cell precursors promotes the development of medulloblastoma. Heterozygous deletion of the murine homologue of PTCH1 (Ptc1) results in medulloblastoma in approximately one third of mice by 25 weeks of age.7 Granule-cell precursors in the external granule-cell layer give rise to these tumors, which are associated with loss or mutation of the remaining wild-type Ptc1 allele.8 Inhibition of the hedgehog pathway by SMO inhibitors such as cyclopamine and HhAntag result in regression of medulloblastoma tumors in Ptc1+/− mice.9,10 Constitutive activation of hedgehog signaling — often due to inactivating mutations of PTCH1 — has been shown in approximately 30% of medulloblastomas in humans.11 Medulloblastoma develops in approximately 5% of patients with nevoid basal-cell carcinoma syndrome (Gorlin’s syndrome), an autosomal dominant disorder of germ-line PTCH mutations.12
GDC-0449 is an orally bioavailable selective inhibitor of SMO (Fig. 1A in the Supplementary Appendix, available with the full text of this article at NEJM.org). GDC-0449 induces rapid regression of the tumor and suppression of the hedgehog pathway in the Ptc1+/− murine medulloblastoma model (Fig. 1B and 1C in the Supplementary Appendix).
Preliminary pharmacokinetic, safety, and efficacy data from our ongoing phase 1 clinical trial of daily administration of GDC-0449 in patients with advanced solid tumors have been presented recently.13 Initial results suggest that this agent has an acceptable side-effect profile at doses that lead to steady-state concentrations above those predicted to have efficacy in preclinical models. Preliminary efficacy was seen in patients with advanced basal-cell carcinoma, another type of tumor that is known to harbor PTCH1 mutations.14 The highest dose level of GDC-0449 used in this trial was 540 mg per day.
CASE REPORT
The patient was a 26-year-old man who received the diagnosis of medulloblastoma confined to the cerebellum in 2004, when he was 22 years of age. Initial therapy included gross total resection and adjuvant craniospinal irradiation followed by the administration of carboplatin, etoposide, cyclophosphamide, and vincristine. Recurrent and metastatic disease was confirmed by means of a biopsy 2 years after the initial diagnosis, and the patient was treated with induction etoposide, temozolomide, and cyclophosphamide. After a partial response was achieved, he underwent autologous stem-cell transplantation. Eighteen months later, widespread systemic metastatic disease developed without a recurrence in the central nervous system. The patient underwent two surgeries and two radiosurgical procedures for the management of epidural intraspinal metastatic medulloblastoma. Additional systemic therapies in 2008 included temozolomide, which was discontinued owing to refractory thrombocytopenia, and bevacizumab, which the patient received in March and April of 2008 and which was subsequently discontinued owing to progression of the disease in the context of worsening pancytopenia.
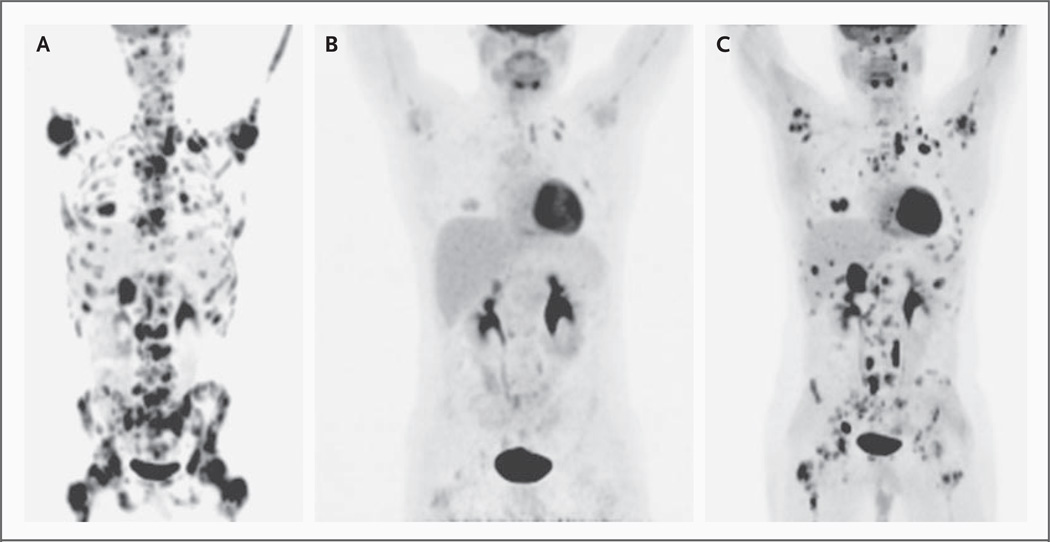
The patient was evaluated in April 2008. He reported diffuse pain in his bones (the severity of which he rated as 5 on a scale of 0 to 10, with higher numbers indicating more severe pain), despite the administration of up to 640 mg of oxycodone daily. On physical examination, the patient was observed to be a thin man with palpable left supraclavicular lymphadenopathy and indurated masses protruding from the sternum. Laboratory findings were notable for pancytopenia (total white cells, 1750 per cubic millimeter; hemoglobin level, 9.7 g per deciliter; and platelets, 14,000 per cubic millimeter). He required transfusions of platelets twice a week and transfusions of packed red cells approximately once a week. A combined positron-emission tomographic (PET) and computed tomographic (CT) scan revealed extensive skeletal and soft-tissue metastases (Fig. 1A).
Figure 1. Tumor Response on Positron-Emission Tomographic (PET) Scanning.
Whole-body projections from 18F-fluorodeoxyglucose (FDG)–PET scans are shown. Panel A shows the pretreatment scan; Panel B, the repeat scan after 2 months of therapy with the hedgehog pathway inhibitor GDC-0449; and Panel C, the repeat scan after 3 months of therapy.
The patient was referred for consideration of enrollment in the phase 1 trial of GDC-0449. He did not meet enrollment criteria owing to the hematologic abnormalities. The pancytopenia was thought to be consistent with extensive marrow involvement. No clinically significant hematologic toxic effects had been observed in the clinical trials of GDC-0449 up to that time. Because of the lack of alternative therapies and the preclinical evidence that hedgehog signaling may be critical in medulloblastoma, a protocol deviation was proposed to allow this patient to be treated with GDC-0449. The protocol deviation was approved by the institutional review board at Johns Hopkins University on April 18, 2008, and the patient provided written informed consent.
Results
The patient received a single oral dose of 540 mg of GDC-0449 on April 22, 2008, followed 1 week later by initiation of daily dosing at 540 mg per day. The delay in the initiation of daily dosing was to allow for pharmacokinetic analyses of a single dose. Over the course of the week after administration of the single dose, his pain increased from 5 on the 10-point scale to 8; the pain was primarily localized in the shoulders. A magnetic resonance imaging (MRI) scan on April 25, 2008, showed matted right supraclavicular lymphadenopathy and an epidural mass at C7, with impending compression of the thecal sac (Fig. 3A and 3B in the Supplementary Appendix). The patient refused to undergo further spinal surgery. Dexamethasone therapy, at a dose of 4 mg twice a day, was started for the management of pain; he received dexamethasone throughout the remainder of his treatment with GDC-0449. The pain lessened over the next 3 days, and the need for oxycodone decreased from a dose of up to 640 mg daily to 320 mg daily.
The patient began receiving 540 mg of GDC-0449 per day on April 29, 2008. On May 19, 2008, the supraclavicular lymphadenopathy and one of two sternal masses were no longer palpable; the other sternal mass was noted by the treating physician to have decreased in size, and he had no pain (0 on the 10-point scale). On June 23, 2008, he had no palpable nodules, continued to have a pain score of 0, and had returned to his normal level of activity. The physical examination was notable for weight gain — from 60 kg at baseline to 67 kg — and the absence of any palpable tumor. The pancytopenia persisted (total white cells, 960 per cubic millimeter; hemoglobin level, 9.3 g per deciliter; and platelets, 10,000 per cubic millimeter). A PET scan revealed marked diminution of 18F-fluorodeoxyglucose (FDG) avidity at all prior sites of disease (Fig. 1B). Measurable disease noted previously, including the supraclavicular lymphadenopathy, was markedly diminished (Fig. 3A and 3C in the Supplementary Appendix). The previously noted epidural mass at C7 was no longer detectable (Fig. 3B and Fig. 3D in the Supplementary Appendix). Over the course of the treatment with GDC-0449, the requirement for platelet transfusions decreased from twice a week to once a week. On July 23, 2008, approximately 3 months after the initiation of therapy with GDC-0449, a confirmatory restaging study was performed; the results indicated a continued response of some lesions but a marked increase in PET activity for several existing and new lesions (Fig. 1C) and evidence of tumor regrowth at some sites (Fig. 3E and 3F in the Supplementary Appendix). The patient was removed from the phase 1 study because of progression of the disease. During the time he was in the study, no adverse events higher than grade 1 that were related to treatment with GDC-0449 were reported. The disease progressed rapidly, despite a series of subsequent therapies, and the patient died on September 23, 2008.
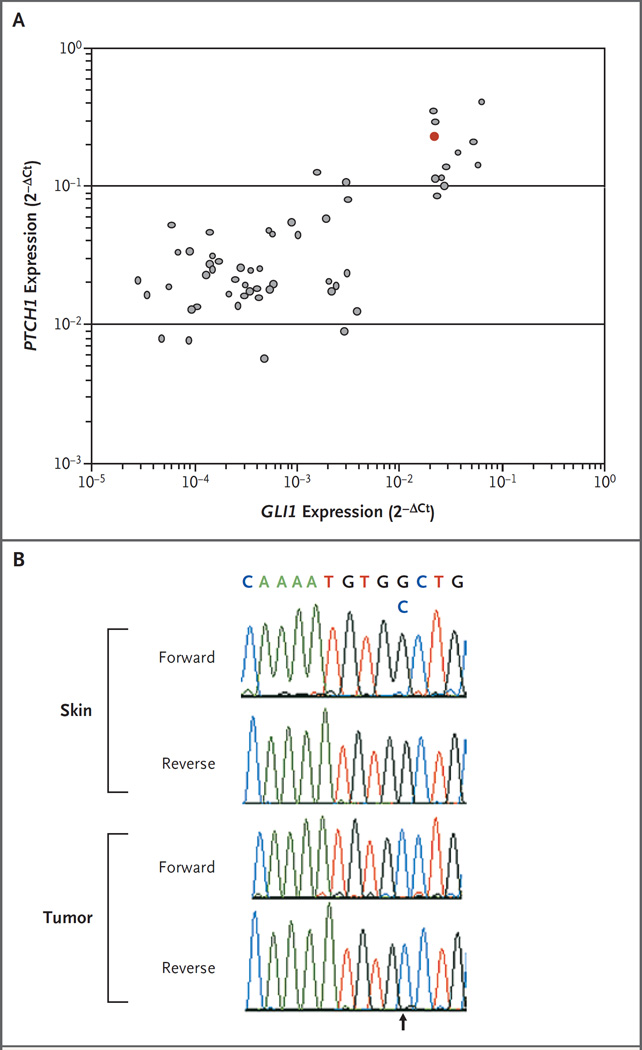
Transcriptional profiling of medulloblastoma for hedgehog pathway target genes has been shown to accurately predict the presence of hedgehog pathway mutations.15 A biopsy specimen of the patient’s metastatic tumor obtained before the initiation of GDC-0449 therapy was evaluated for markers of hedgehog pathway activation. Pathway activation by SMO leads to transcription of downstream target genes, including GLI family zinc finger 1 (GLI1), PTCH1, patched homologue 2 (PTCH2), and secreted frizzled-related protein 1 (SFRP1). Expression of these target genes in this patient’s tumor was compared with the expression in a panel of 55 archival medulloblastoma samples by means of quantitative reverse-transcriptase–polymerase-chain-reaction (RT-PCR) (see Methods in the Supplementary Appendix). Expression of all four genes was in the upper 20% of all tumors evaluated, a finding that was suggestive of active hedgehog pathway signaling (Fig. 2A, and Fig. 2 in the Supplementary Appendix). Genomic analysis of the PTCH1 locus in tumor cells was consistent with loss of heterozygosity and a single nucleotide substitution in exon 15 causing a W→C amino acid change at position 844 (Fig. 2B; for further details, see Methods in the Supplementary Appendix). Somatic mutations in the same exon, encoding the second extracellular loop domain of PTCH1, have been described previously and are summarized in the PTCH mutation database (http://www.cybergene.se/cgi-bin/w3-msql/ptchbase/index.html). DNA from a biopsy specimen of normal skin revealed a wild-type sequence and no loss of heterozygosity, confirming that these alterations were somatic (Fig. 2B). Sequencing of genes encoding other members of the hedgehog pathway revealed no nonsynonymous sequence alterations in suppressor of fused homologue (SuFu) or SMO (data not shown).
Figure 2. Tumor-Specific Hedgehog Pathway Activation.
Panel A shows the expression of GLI family zinc finger 1 (GLI1) and patched homologue 1 (PTCH1) messenger RNA in the patient’s tumor (red dot) relative to the expression in a panel of 55 banked medulloblastoma samples (gray dots). GLI1 and PTCH1 expression levels were assessed with the use of real-time polymerase-chain-reaction assays, and results are presented as normalized gene expression (2−ΔCt). Panel B shows the nucleotide sequence of the PTCH1 gene in specimens of the patient’s skin and tumor. In the tumor specimen, both forward and reverse reactions show a homozygous mutation at position 2720, resulting in a G→C change (arrow).
Discussion
We describe a patient with advanced medulloblastoma that had been refractory to multiple prior therapies. The tumor had a remarkable, although incomplete, and rapid, although transient, response to inhibition of the hedgehog pathway with GDC-0449. To our knowledge, this report marks the first demonstration that an inhibitor of the hedgehog pathway can cause regression of medulloblastoma. The regression is notable because of the tumor burden and the extent of metastasis in this patient, with substantial soft-tissue and bony-tissue involvement and clinically significant bone marrow compromise, and underscores the primary role that the hedgehog pathway played in maintaining and driving the growth of this patient’s tumor.
Our observation can be placed within the larger context of recent progress in cancer treatment involving targeted inhibitors of mutant signaling pathways. Frequently cited examples of targeting tumor-specific mutations include the use of imatinib in patients with chronic myelogenous leukemia and gastrointestinal stromal tumors, and the use of erlotinib in patients with non–small-cell lung cancer.16–18 In these examples, targeted inhibition of mutant and dysfunctional tyrosine kinases that have been implicated in the development, proliferation, and survival of tumor cells leads to remarkable antitumor responses. The case we describe represents a variation on this theme: a small-molecule inhibitor that targets an intact signaling factor (SMO), which is the primary target of a mutated and dysfunctional tumor suppressor (PTCH1).
A minority of medulloblastoma tumors are thought to harbor PTCH1 mutations or to be driven in some other way by hedgehog pathway signaling.19 The development of a diagnostic marker for hedgehog pathway activation has been challenging because alteration of many pathway components may result in an activated phenotype. On the basis of a recently published gene-expression signature that appears to correlate with hedgehog pathway activation in medulloblastoma,15 this patient’s tumor showed specific pathway activation. Testing this and other potential strategies for identifying biomarkers will be important components of future clinical trials of hedgehog pathway inhibitors.
Even complete or near-complete responses to imatinib and erlotinib may not be durable. Multiple mechanisms of acquired resistance have been described, including secondary mutations in the kinase targets and up-regulation of alternative tyrosine kinases.20–23 Similarly, after initial marked regression, the tumor in our patient rapidly acquired resistance. Identifying the mechanisms of acquired resistance to selective hedgehog pathway inhibitors in patients with medulloblastoma will be of particular interest in future studies.
The use of a targeted inhibitor of a pathway that is clearly implicated in malignant transformation in medulloblastoma may offer a more effective therapeutic option than treatments that are currently available and may avoid some of their adverse effects. The hedgehog pathway regulates many developmental processes. Although an acceptable side-effect profile has been observed in an initial cohort of adults, it is unclear what the adverse effects of hedgehog pathway blockade may be in prepubescent children. Skeletal growth complications, including effects on both cartilage and bone formation, may limit the use of these therapies in young children.24 Cautious application of these initial observations through carefully monitored clinical trials involving a broad spectrum of patients with medulloblastoma is warranted.
Supplementary Material
Acknowledgments
Supported by Genentech. GDC-0449 was developed under a collaborative agreement between Genentech and Curis. Genentech and Roche collaborate on the clinical development of GDC-0449. Also supported by a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund (to Dr. Rudin).
Drs. Rudin, Von Hoff, and LoRusso report receiving research funding from Genentech for a GDC-0449 phase 1 trial; Dr. Rudin, receiving a BioOncology Grant Program Award from Genentech; Dr. Laterra, receiving a consulting fee from Genentech; Dr. LoRusso, receiving lecture fees from Genentech; Drs. Yauch, Callahan, Fu, Gould, Holcomb, Stinson, de Sauvage, and Low, being employees of Genentech; and Dr. de Sauvage, holding patents in the area of hedgehog signaling.
We thank Dr. Richard Gilbertson for advice and discussion on molecular profiling of medulloblastoma.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 2.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 3.Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 4.Fan X, Eberhart CG. Medulloblastoma stem cells. J Clin Oncol. 2008;26:2821–2827. doi: 10.1200/JCO.2007.15.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 6.Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–9067. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 8.Oliver TG, Read TA, Kessler JD, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 9.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 10.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Miller HL, Jensen P, et al. A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63:5428–5437. [PubMed] [Google Scholar]
- 12.Cowan R, Hoban P, Kelsey A, Birch JM, Gattamaneni R, Evans DG. The gene for the naevoid basal cell carcinoma syndrome acts as a tumour-suppressor gene in medulloblastoma. Br J Cancer. 1997;76:141–145. doi: 10.1038/bjc.1997.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudin CM, Von Hoff DD, LoRusso PM, et al. Updated safety and efficacy data from a first-in-human, first-in-class phase I study of Hedgehog pathway antagonist GDC-0449. Eur J Cancer. 2008;6:112. abstract. [Google Scholar]
- 14.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 15.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 16.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 17.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 18.Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol. 2005;23:3227–3234. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 19.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 21.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian HM, Talpaz M, Giles F, O’Brien S, Cortes J. New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Ann Intern Med. 2006;145:913–923. doi: 10.7326/0003-4819-145-12-200612190-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.