Abstract
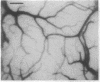
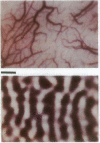
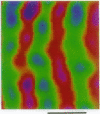
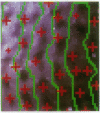
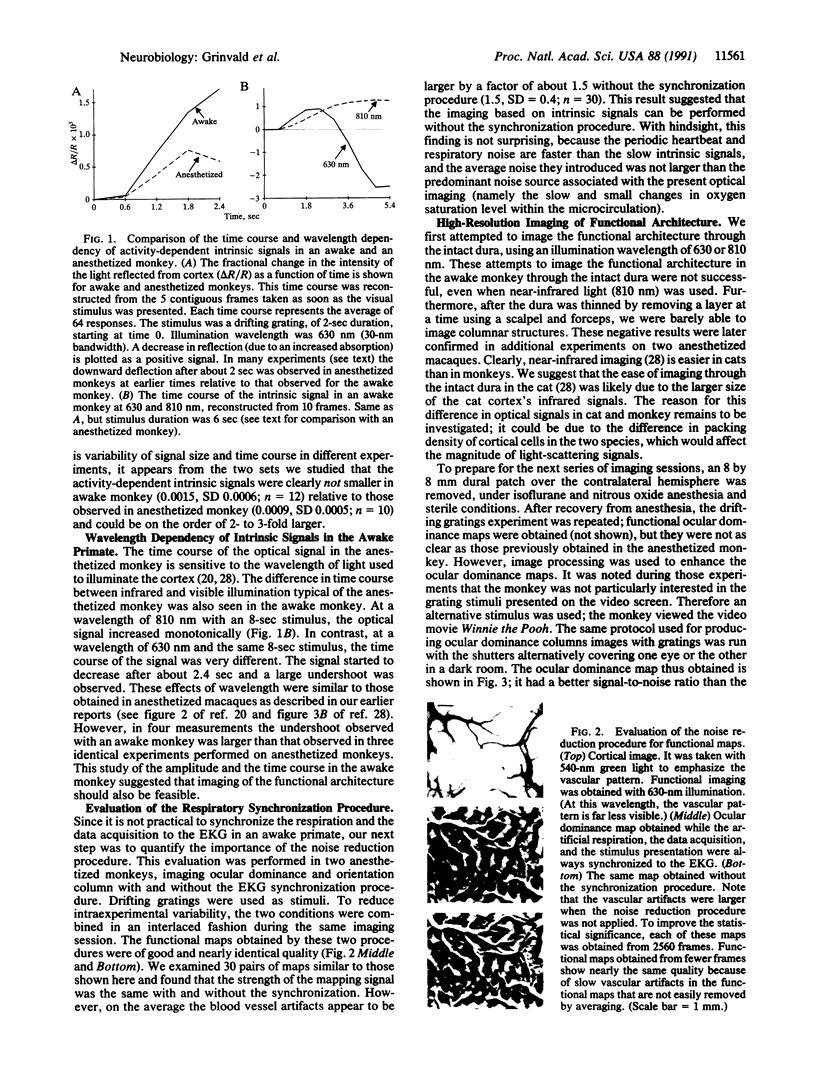
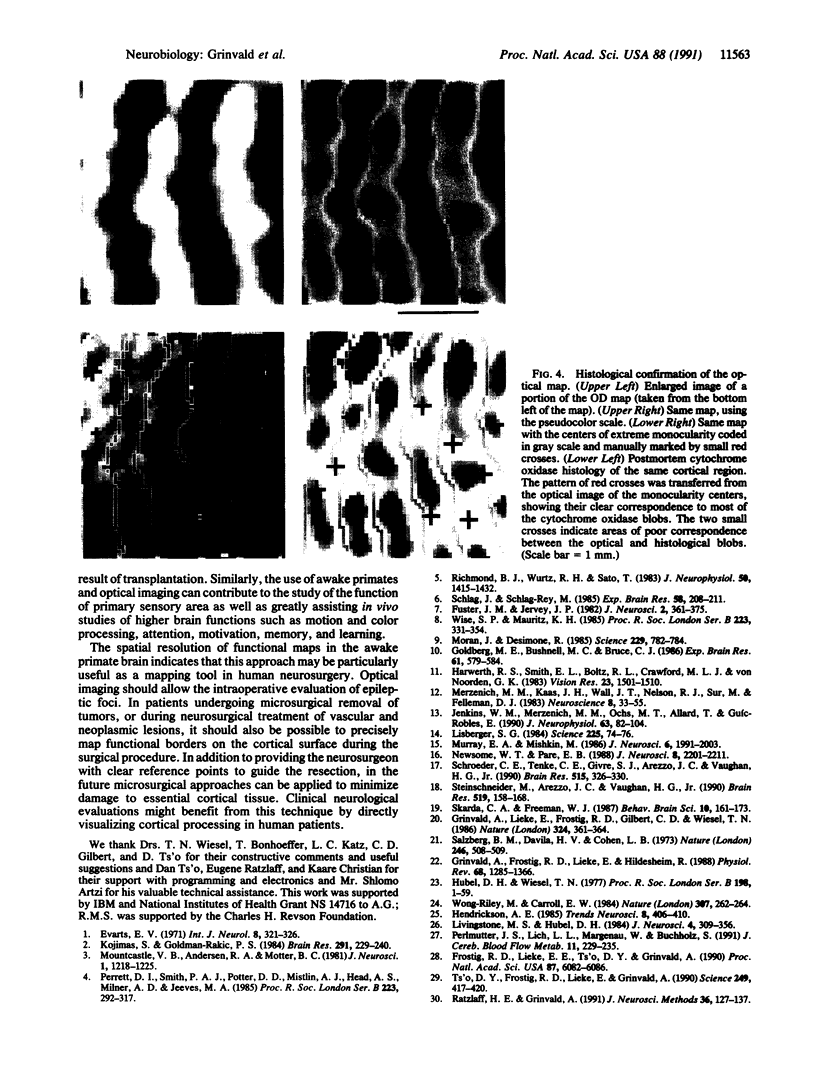
Optical imaging of the functional architecture of cortex, based on intrinsic signals, is a useful tool for the study of the development, organization, and function of the living mammalian brain. This relatively noninvasive technique is based on small activity-dependent changes of the optical properties of cortex. Thus far, functional imaging has been performed only on anesthetized animals. Here we establish that this technique is also suitable for exploring the brain of awake behaving primates. We designed a chronic sealed chamber and mounted it on the skull of a cynomolgus monkey (Macaca fascicularis) over the primary visual cortex to permit imaging through a transparent glass window. Restriction of head position alone was sufficient to eliminate movement noise in awake monkey imaging experiments. High-resolution imaging of the ocular dominance columns and the cytochrome oxidase blobs was achieved simply by taking pictures of the exposed cortex when the awake monkey was viewing video movies alternatively with each eye. Furthermore, the functional maps could be obtained without synchronization of the data acquisition to the animal's respiration and the electrocardiogram. The wavelength dependency and time course of the intrinsic signal were similar in anesthetized and awake monkeys, indicating that the signal sources were the same. We therefore conclude that optical imaging is well suited for exploring functional organization related to higher cognitive brain functions of the primate as well as providing a diagnostic tool for delineating functional cortical borders and assessing proper functions of human patients during neurosurgery.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evarts E. V. Activity of thalamic and cortical neurons in relation to learned movement in the monkey. Int J Neurol. 1971;8(2):321–326. [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. M., Jervey J. P. Neuronal firing in the inferotemporal cortex of the monkey in a visual memory task. J Neurosci. 1982 Mar;2(3):361–375. doi: 10.1523/JNEUROSCI.02-03-00361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. E., Bushnell M. C., Bruce C. J. The effect of attentive fixation on eye movements evoked by electrical stimulation of the frontal eye fields. Exp Brain Res. 1986;61(3):579–584. doi: 10.1007/BF00237584. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Frostig R. D., Lieke E., Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988 Oct;68(4):1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Harwerth R. S., Smith E. L., 3rd, Boltz R. L., Crawford M. L., von Noorden G. K. Behavioral studies on the effect of abnormal early visual experience in monkeys: spatial modulation sensitivity. Vision Res. 1983;23(12):1501–1510. doi: 10.1016/0042-6989(83)90162-1. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Jenkins W. M., Merzenich M. M., Ochs M. T., Allard T., Guíc-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990 Jan;63(1):82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Kojima S., Goldman-Rakic P. S. Functional analysis of spatially discriminative neurons in prefrontal cortex of rhesus monkey. Brain Res. 1984 Jan 23;291(2):229–240. doi: 10.1016/0006-8993(84)91255-1. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G. The latency of pathways containing the site of motor learning in the monkey vestibulo-ocular reflex. Science. 1984 Jul 6;225(4657):74–76. doi: 10.1126/science.6610214. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984 Jan;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J., Nelson R. J., Sur M., Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983 Jan;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Moran J., Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985 Aug 23;229(4715):782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Andersen R. A., Motter B. C. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci. 1981 Nov;1(11):1218–1225. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. A., Mishkin M. Visual recognition in monkeys following rhinal cortical ablations combined with either amygdalectomy or hippocampectomy. J Neurosci. 1986 Jul;6(7):1991–2003. doi: 10.1523/JNEUROSCI.06-07-01991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W. T., Paré E. B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci. 1988 Jun;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter J. S., Lich L. L., Margenau W., Buchholz S. PET measured evoked cerebral blood flow responses in an awake monkey. J Cereb Blood Flow Metab. 1991 Mar;11(2):229–235. doi: 10.1038/jcbfm.1991.54. [DOI] [PubMed] [Google Scholar]
- Perrett D. I., Smith P. A., Potter D. D., Mistlin A. J., Head A. S., Milner A. D., Jeeves M. A. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Ratzlaff E. H., Grinvald A. A tandem-lens epifluorescence macroscope: hundred-fold brightness advantage for wide-field imaging. J Neurosci Methods. 1991 Feb;36(2-3):127–137. doi: 10.1016/0165-0270(91)90038-2. [DOI] [PubMed] [Google Scholar]
- Richmond B. J., Wurtz R. H., Sato T. Visual responses of inferior temporal neurons in awake rhesus monkey. J Neurophysiol. 1983 Dec;50(6):1415–1432. doi: 10.1152/jn.1983.50.6.1415. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Davila H. V., Cohen L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973 Dec 21;246(5434):508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Schlag J., Schlag-Rey M. Unit activity related to spontaneous saccades in frontal dorsomedial cortex of monkey. Exp Brain Res. 1985;58(1):208–211. doi: 10.1007/BF00238971. [DOI] [PubMed] [Google Scholar]
- Schroeder C. E., Tenke C. E., Givre S. J., Arezzo J. C., Vaughan H. G., Jr Laminar analysis of bicuculline-induced epileptiform activity in area 17 of the awake macaque. Brain Res. 1990 May 7;515(1-2):326–330. doi: 10.1016/0006-8993(90)90617-k. [DOI] [PubMed] [Google Scholar]
- Steinschneider M., Arezzo J. C., Vaughan H. G., Jr Tonotopic features of speech-evoked activity in primate auditory cortex. Brain Res. 1990 Jun 11;519(1-2):158–168. doi: 10.1016/0006-8993(90)90074-l. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Frostig R. D., Lieke E. E., Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990 Jul 27;249(4967):417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- Wise S. P., Mauritz K. H. Set-related neuronal activity in the premotor cortex of rhesus monkeys: effects of changes in motor set. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):331–354. doi: 10.1098/rspb.1985.0005. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M., Carroll E. W. Effect of impulse blockage on cytochrome oxidase activity in monkey visual system. Nature. 1984 Jan 19;307(5948):262–264. doi: 10.1038/307262a0. [DOI] [PubMed] [Google Scholar]