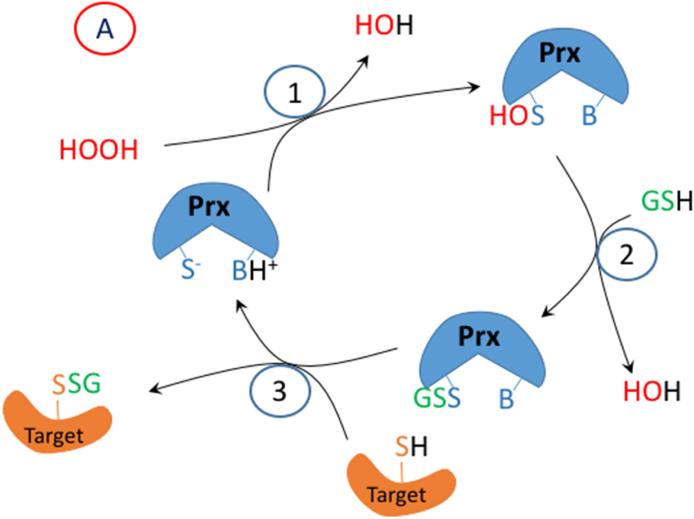
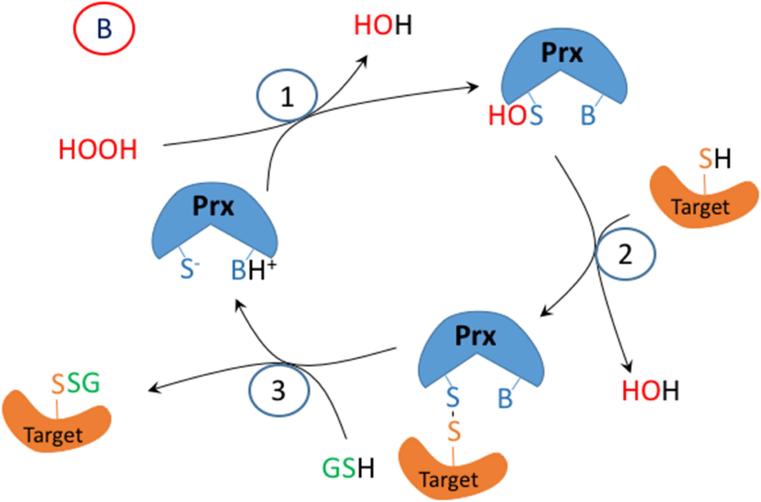
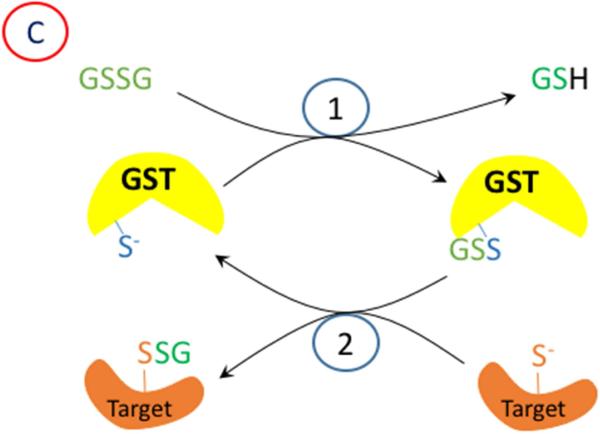
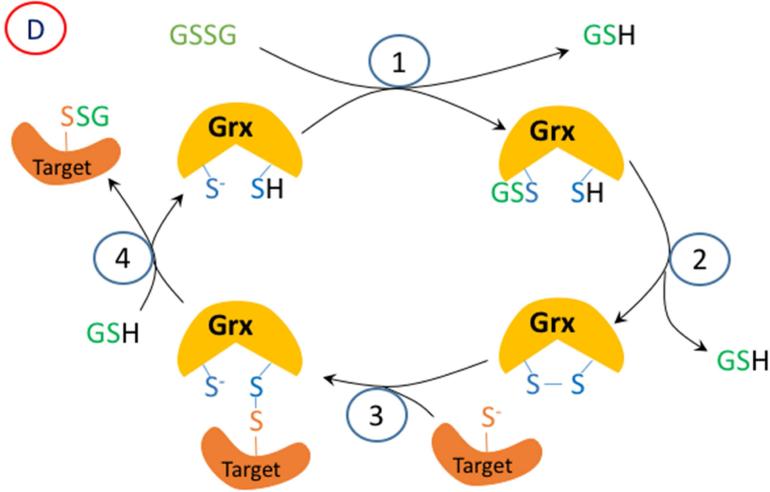
Figure 2.
A proposed mechanism for enzymatic glutathionylation of target signaling proteins by peroxiredoxin (Prx), glutathione S-transferase π (GST), or glutaredoxin (Grx). Similar reactions may also occur with GPx. In (A), 1- H2O2 oxidized Prx; 2- GSH forms a disulfide with Prx; 3- disulfide exchange occurs with the target protein thiolate. In (B), steps 2 and 3 are in reverse sequence from (A). In (C), 1- GSSG, which must be formed by a closely associated peroxidase undergoes disulfide exchange with the thiolate in the active site of GST π; 2- disulfide exchange occurs with the target protein thiolate. In (D), GSSG, which must be formed by a closely associated peroxidase undergoes disulfide exchange with the thiolate in the active site of Grx; 2- An intramolecular disulfide forms in the active site of Grx; 3- disulfide exchange occurs with the target protein thiolate; 4- disulfide exchange occurs with GSH.