Abstract
Background
Atopic dermatitis (AD) is an inflammatory skin disorder with severe pruritus. Despite advancements in medicine, therapeutic treatments for AD are still limited. Eupatilin (5,7-dihydroxy-30,40,6-trimethoxyflavone) is one of the lipophilic flavonoids from Artemisia umbelliformis Lam. and Artemisia genipi Weber.
Objective
Although it has been reported to act a role in improving inflammation, its action on AD is uncertain. In this study, we examined the role of eupatilin on AD-like skin lesions in NC/Nga mice.
Methods
2,4-dinitrochlorobenzene was repeatedly applied to the ear of NC/Nga mice to produce AD-like skin lesions. Eupatilin (1%, once a day for 5 consecutive days/week) was applied topically for four weeks for the evaluation of its therapeutic effects.
Results
1% eupatilin cream significantly reduced the clinical severity score of AD-like lesions, compared to the vehicle (p<0.005). A histopathological analysis revealed that 1% eupatilin cream significantly decreased the mast cell infiltration as well as inflammatory cell infiltration, compared to the vehicle (p<0.005). We showed that 1% eupatilin cream significantly reduced the expression of thymic stromal lymphopoietin, tumor necrosis factor-α, interleukin-4, and interleukin-19, but not interferon-γ, compared to the vehicle (p<0.005).
Conclusion
Considering the therapeutic reaction of eupatilin on AD-like lesions as in this study, the substance has a promising to be an adjuvant topical agent for the control of AD.
Keywords: Atopic dermatitis, Dermatitis, Eupatilin, Interleukin-4, NC/Nga mice
INTRODUCTION
Atopic dermatitis (AD) is a common chronic inflammatory skin disease1. It is characterized by intense pruritus and relapsing eczema. Although the pathomechanisms of AD is not yet fully elucidated, studies on its pathogenesis have demonstrated that the disease is highly associated with the dysregulation of the immune system, in particular, T-helper 2 (Th2)–immune responses. Thymic stromal lymphopoietin (TSLP) as well as Th2 cell producing interleukin (IL)-4, IL-5 and IL-13 play main roles in the pathogenesis of AD. Recent progress has revealed that IL-19 is up-regulated by IL-4 related in the pathogenesis of AD2. Although Th2 cells are principal during the acute reaction of AD, Th1 cells producing interferon (IFN)-γ are highly expressed during the chronic AD phase3,4. Until now, Local or systemic glucocorticosteroids as well as emollient is the main therapeutic modalities for control of AD5,6. However, there have been controversies regarding their long-term use, because these steroids can produce side effects in AD patients7. Therefore, many researchers are trying to develop a more effective and safer topical agent for AD.
Eupatilin (2-[3,4-dimethoxyphenyl]-5,7-dihydroxy-6-methoxychromen-4-one), is the main lipophilic flavonoid obtained from medicinal herbs of the genus Artemisia, such as Artemisia umbelliformis Lam. and Artemisia genipi Weber. Eupatilin has cytoprotective and anti-apoptotic activities in gastric epithelial primary cells, and anti-proliferative activities in cancer cells8,9,10. It has been also shown to act as an antioxidant by inhibiting reactive oxygen species and has anti-inflammatory activities such as the inhibition of 5-lipoxygenase8. Recently, some reports showed that eupatilin can inhibit the allergic effects of mast cells11,12. Moreover, it inhibits the expression of the proinflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-4, IL-6, and IL-18 through nuclear factor kappa B11. However, so far, no study has assessed the therapeutic effects and mechanisms of the topical application of eupatilin on AD murine models.
AD animal model is spontaneously induced in the NC/Nga mouse13. A previous report showed that 2,4-dinitrochlorobenzene (DNCB), an electrophilic and cytotoxic benzene derivative, induces AD-like skin diseases in NC/Nga mice14. In this study, we aimed to examine the effect of topical eupatilin on AD-like cutaneous lesions in NC/Nga mouse. The result of topical eupatilin in AD-like NC/Nga mice was evaluated by counting the skin severity score and detecting histological changes of lesions and levels of mRNA expression of Th2 cytokines.
MATERIALS AND METHODS
All animal care was performed in accordance with the Guide for the Care and Use of Laboratory Animals (Washington, DC, USA) and was approved by the Animal Care and Use Committee of The Catholic University of Korea (2014-0172-01).
Animals and reagents
Four-week-old male NC/Nga mice were purchased from SLC Japan (Tokyo, Japan). Mice were maintained under specific pathogen-free conditions at 24℃±1℃ with humidity of 50%±10% under a 12-hour light-dark cycle. Food and tap water were provided ad libitum15. 1% eupatilin was prepared by diluting eupatilin into the vehicle as previously described15. The vehicle included glycerin (5%), xanthan gum (0.1%), 1,3-butylene glycol (6.0%), carbomer (0.3%), beeswax (2.0%), cetanol (2.0%), white petrolatum (1.5%), lecithin (0.5%), arlacel 165 (2.0%), tween 60 (0.2%), arlacel 60 (0.2%), liquid paraffin (6.0%), dimethicone (1.0%), triethanolamine 0.3%, preservative (0.3%), and water (up to 100.0%)15. Eupatilin was kindly given by Dong-A Pharmaceutical (Seoul, Korea). 1% eupatilin cream was prepared by mixing eupatilin with vehicle by COTDE Co., Ltd. (Cheonan, Korea). 0.03% tacrolimus ointment (Protopic®) was got from Astellas Pharma Inc. (Tokyo, Japan).
Induction of AD-like skin lesions in NC/Nga mice and topical application of eupatilin
DNCB-induced AD-like skin lesions in NC/Nga mice were induced as described in the previous study14. To produce AD-like cutaneous lesions, DNCB was treated to the abdomens and ears of mice. For sensitization, 150 µl of 1% DNCB dissolved in 4:1 acetone:olive oil was epicutaneously applied to the abdomen once (day 7). One week after the sensitization (day 1), 20 µl of 0.4% DNCB was treated to the dorsal surface of each ear. The 0.4% DNCB was applied repeatedly to the ears every other day for four weeks, and then five times a week for a further four weeks along with other topical treatments15.
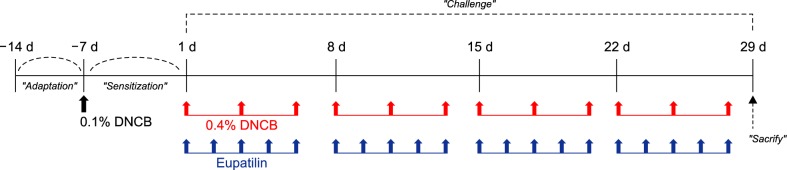
The experimental design is shown in Fig. 1. Mice were divided into four groups (n=5 per group): (1) non-treated (NT) group, (2) DNCB+vehicle (VH), (3) DNCB+1% eupatilin cream (0.05 g per one ear), and (4) DNCB+0.03% tacrolimus ointment (0.05 g per one ear). The clinical severity score (erythema/hemorrhage, edema, excoriation/erosion and scaling/dryness) of AD was assessed. Each item was rated from 0 (none), 1 (mild), 2 (moderate), to 3 (severe). Ear thickness was measured once per week using dial calipers (Kori Seiki MFG, Tokyo, Japan). Two investigators in a blinded fashion independently performed the assessment15.
Fig. 1. Experimental design. DNCB: 2,4-dinitrochlorobenzene.
Histological examination
We evaluated the histological findings of the ear skin of each mouse whose sections were stained with hematoxylin and eosin (H&E). The ear skin of each mouse was prepared on the last day of the experiment (day 29), fixed with 4% formalin, and embedded in paraffin. Then, 5-µm-thick sections were cut and transferred onto slides. Deparaffinized skin sections were stained with H&E before they were examined at 100× magnification. We also investigated the number of mast cells in the skin with staining with toluidine blue (TB). To detect mast cell infiltration, the dorsal skin of each mouse was also prepared on the last day of the experiment (day 29) as described above. All deparaffinized skin sections were stained with TB. Two researchers independently conuted the number of mast cells in five randomly selected sites at 400× magnification.
Quantitative reverse transcription-polymerase chain reaction (QRT-PCR)
Ear skin was collected on the last day of the experiment (day 29) and stored at −80℃ until use. Total RNA was isolated using Trizol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol15. Equal amounts of RNA (1 µg) were reverse transcribed into cDNA using Prime Script™ real-time (RT) reagent Kit with gDNA Eraser® (Takara Bio, Otsu, Japan). Quantitative RT-PCR was performed using a CFX96™ Real-Time PCR Detection System® (Bio-Rad, Hercules, CA, USA) with SYBR® premix EX Taq™ (Takara Bio) and specific primer for TSLP, IL-4, IL-5, IL-13, IL-19, TNF-α, and IFN-γ15. The following PCR primers were used for real-time PCR: TSLP (NM_02136), IL-4 (NM_021283), IL-5 (NM_010558), IL-13 (NM_008355), IL-19 (NM_001009940), TNF-α (NM_013693), and IFN-γ (NM_008337). The cycling conditions consisted of an initialization step for ten sec at 95℃ followed by two-step PCR for 45 cycles of 95℃ for five seconds (denaturation) and 58℃~60℃ for 30 seconds (annealing/extension)15. Fluorescence intensity was measured in real time using the optical module15. Melt curves were used to determine product specificity. Results were normalized to the level of β-actin gene expression15. The analysis of relative gene expression data was conducted using the 2−ΔΔCT method15. Experiments were examined two times.
Statistical analysis
All data were expressed as the mean±standard error of the mean. One-way or two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test was used for statistical analysis15. In comparisons of the three groups, we used the Kruskal-Wallis test . Differences were considered significant at p<0.05. Statistical analyses were performed using Prism 5 (Graphad Software, San Diego, CA, USA).
RESULTS
Effect of topical 1% eupatilin cream on DNCB-induced AD-like skin lesions of NC/Nga mice
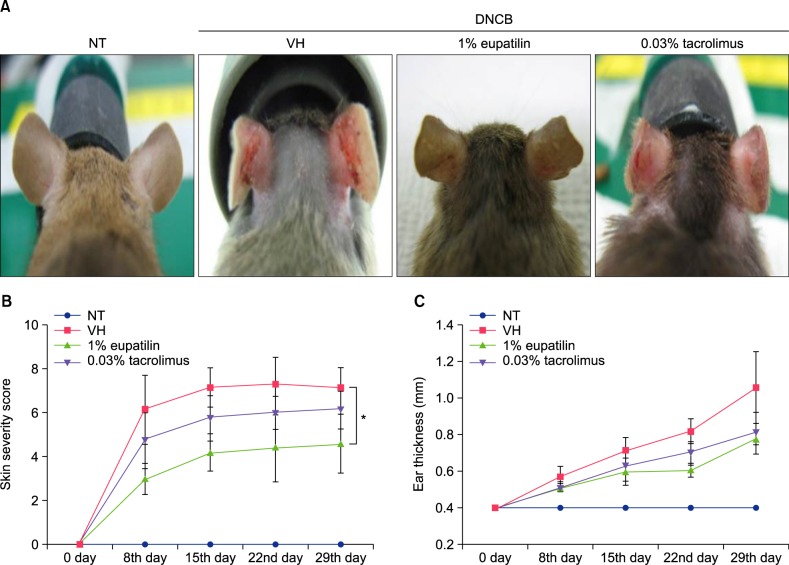
To investigate the effects of eupatilin on an AD murine model, inflammation was induced on the ears of Nc/Nga mice by DNCB applied three times a week. Topical applications of DNCB on the ears of NC/Nga mice produce AD-like lesions including edematous erythema with scratching mark and dryness (Fig. 2A). The clinical severity grades of the DNCB+VH group gradually escalated after the first trial. On day 29 after the first trial, the clinical severity ratings dropped by 33% and 15% in the DNCB+1% eupatilin cream and DNCB+0.03% tacrolimus ointment group, individually, compared to the DNCB+VH group (Fig. 2B). Treatment with 1% eupatilin significantly reduced DNCB-induced clinical severity score on day 29, compared to the DNCB+VH group (p<0.05). Treatment with 1% eupatilin and 0.03% tacrolimus reduced DNCB-induced ear hyperplasia on day 29 by 26% and 23%, respectively, compared to the DNCB+VH group (Fig. 2C).
Fig. 2. (A) Photographs of the clinical features of NC/Nga mice non-treated (NT), 2,4-dinitrochlorobenzene (DNCB)+vehicle (VH), DNCB+1% eupatilin cream, DNCB+0.03% tacrolimus ointment, on day 29 following the first challenge. (B) Clinical skin severity scores. (C) Ear thickness. Photos and graphs revealed that 1% eupatilin cream significantly reduced the clinical signs such as erythema, edema, excoriation, and dryness on day 29, compared to the vehicle only (p<0.05). Data are presented as mean±standard error of the mean (n=5). *p<0.05, compared to the vehicle group.
Effect of topical 1% eupatilin cream on DNCB-induced epidermal hyperplasia and mast cell infiltration
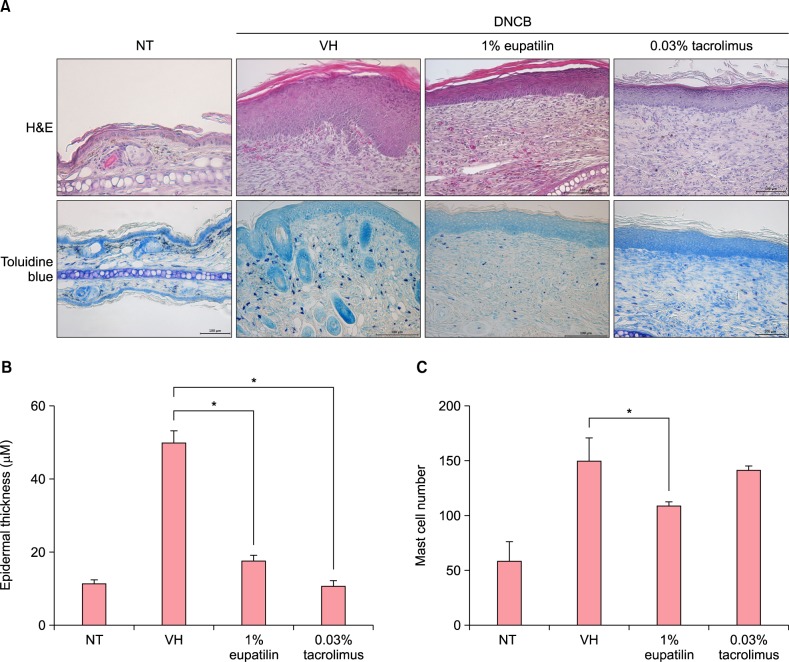
In Fig. 3A, striking epidermal hyperplasia and inflammatory cell infiltration were shown in the dermis of NC/Nga mice, following DNCB (in the DNCB+VH group). In contrast, 1% eupatilin cream reduced the hyperkeratosis, epidermal or dermal thickening and inflammatory cells infiltration in the lesion. The epidermal thickness of 1% eupatilin group significantly reduced, compared to the DNCB+VH group (p<0.05) (Fig. 3B). Kim et al.12 reported that eupatilin has a suppressive effect on the mediator release action of mast cells. We further examined the effect of eupatilin on the infiltrated mast cells. In Fig. 3C, application with DNCB induced infiltrated dermal mast cells. 1% eupatilin significantly reduced infiltrated mast cells, compared to the vehicle (p<0.05). We didn't notice any significant difference in the number of mast cells between groups treated with 1% eupatilin and 0.03% tacrolimus.
Fig. 3. (A) Histological features of atopic dermatitis (AD)-like skin lesions after treatment with 1% eupatilin. 1% eupatilin cream significantly reduced inflammatory cell infiltration (p<0.05) (H&E, ×200) and also significantly reduced mast cell infiltration in the dermis, compared to the vehicle (p<0.05) (toluidine blue, ×200). (B) The epidermal thickness in five randomly chosen visual fields at ×200 magnification (H&E, ×200). (C) The number of mast cells in five randomly chosen visual fields at ×400 magnification (toluidine blue, ×400). Non-treated (NT), DNCB+vehicle (VH), DNCB+1% eupatilin cream, DNCB+0.03% tacrolimus ointment at day 29 following the first challenge. Data are presented as mean±standard error of the mean (n=5). *p<0.05, compared to the vehicle group.
Effect of topical 1% eupatilin cream on DNCB-induced mRNA expression of inflammatory cytokines
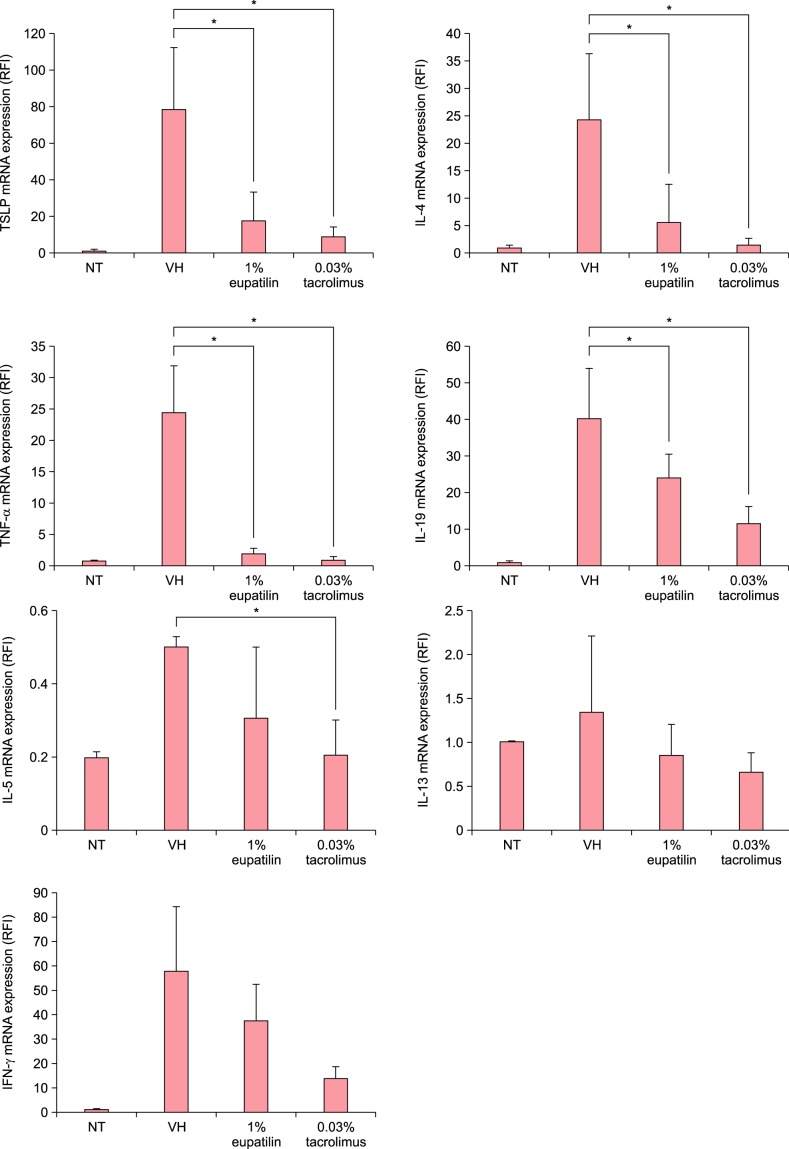
The expression of TNF-α, IL-4, IL-19, and IFN-γ was minimal in the NT group. However, their expression was significantly elevated following DNCB. The application of 1% eupatilin cream significantly reduced the expression of TSLP, TNF-α, IL-4, and IL-19 compared to the vehicle (p<0.05; Fig. 4). The application of 1 % eupatilin cream couldn't significantly decrease the expression of IL-5 and IL-13 (data not shown). DNCB-treated IFN-γ expression of mRNA was also decreased by 1% eupatilin cream, but it was not significant compared to the vehicle.
Fig. 4. Effects of 1% eupatilin on mRNA expression of thymic stromal lymphopoietin (TSLP), tumor necrosis factor (TNF)-α, interleukin (IL)-4, IL-5, IL-13, IL-19, and interferon (IFN-γ) in the ears of NC/Nga mice. 1% eupatilin cream also significantly decreased the mRNA expression of TSLP, TNF-α, IL-4, and IL-19, but not of IL-5, IL-13, and IFN-γ, compared to the vehicle (p<0.05). Non-treated (NT), 2,4-dinitrochlorobenzene (DNCB)+vehicle (VH), DNCB+1% eupatilin cream, DNCB+0.03% tacrolimus ointment. Data are presented as mean±standard error of the mean (n=5). *p<0.05, compared to the vehicle (VH) group. RFI: relapse-free interval.
DISCUSSION
Eupatilin takes in anti-inflammatory, anti-allergic and anti-cancer properties as well as mucosal protective effects16. Here, we showed for the first time that 1% eupatilin cream improved skin lesions of NC/Nga mice. Clinical analyses showed edematous erythema, with excoriation and dryness in the DNCB+VH group, whereas 1% eupatilin cream alleviated these lesional changes. Histologically, 1% eupatilin cream decreased acanthosis, hyperkeratosis and infiltration of inflammatory cells in the skin. Real time RT-PCR revealed significantly reduced levels of the expressed TNF-α, IL-4, IL-19, and TSLP. In addition, infiltration of mast cells was markedly reduced by 1% eupatilin cream.
Recently, several reports have suggested the therapeutic efficacy of eupatilin for various immunologic and inflammatory disorders. Kim et al.12 reported that eupatilin has a suppressive effect on the mediator release of mast cells. Mast cell is an important cell in the progress of numerous allergic diseases including AD17,18. In this study, 1% eupatilin cream largely suppressed the infiltration of mast cells in the DNCB+VH group. We speculated that eupatilin has inhibitory effects on mast cells.
TSLP, mainly from keratinocytes, causes dendritic cells (DC)-mediated immune reactions19. TSLP produces the Th2 response with production of IL-4, IL-5, and IL-13 as well as secretion of TNF-α20. Therefore, TSLP is a mediator that exacerbates the pathogenesis of AD through the initiation of allergic reactions. Our results suggest that 1% eupatilin cream can improve the skin lesions of AD by reducing the elevated expression of TSLP, one of the key mediators in the initiation of AD lesions.
Proinflammatory cytokines, such as TNF-α, by epidermal keratinocyte cells step in the commencement of AD. During the early stage, proinflammatory cytokines activate chemokines and adhesion molecules which initiate the role of leukocytes in the lesion21. In this study, application of 1% eupatilin cream significantly suppressed the TNF-α mRNA expression, which seems to affect inflammatory cell recruitment in the lesion.
Kitagaki et al.18 suggested that T cells and mast cells largely produced Th2 cytokines including IL-4 during acute phase of AD. Additionally, other Th2-cytokines including IL-5 and IL-13, are linked with AD18. Interestingly, IL-19 has been known as one of the Th2 cytokines. Recently Bao et al.22 showed that IL-4 upregulates IL-19 expression in keratinocytes. In addition, Myles et al.23 reported that IL-19 is associated with inflammatory skin diseases including AD. They suggested that IL-19 is possibly a therapeutic target to alter the susceptibility to infection. Th1-type cytokines, such as IFN-γ, were shown to be produced in AD lesions in later stages24,25. We showed that 1% eupatilin cream more effectively decreased the expression of Th2 cytokines (IL-4 and IL-19) than that of Th1 cytokines such as IFN-γ. However, in this study, 1% eupatilin cream couldn't decrease the level of IL-5 or IL-13 in the AD-like lesions.
Overall, these findings suggest that the therapeutic effects of topical 1% eupatilin cream on AD-like skin lesions in NC/Nga mice are possibly mediated by proinflammatory and Th2-response modification.
In conclusion, these findings indicate that topical 1% eupatilin alleviates DNCB-treated AD-like skin lesions in NC/Nga mice. Therefore, we propose that eupatilin may be thought about a promising topical therapeutic candidate in the control of AD. Nonetheless, further studies are needed to show therapeutic mechanisms of 1% eupatilin cream on AD.
ACKNOWLEDGMENT
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C2116). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2015R1C-1A2A01054767).
References
- 1.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao L, Shi VY, Chan LS. IL-4 up-regulates epidermal chemotactic, angiogenic, and pro-inflammatory genes and down-regulates antimicrobial genes in vivo and in vitro: relevant in the pathogenesis of atopic dermatitis. Cytokine. 2013;61:419–425. doi: 10.1016/j.cyto.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Leung DY, Soter NA. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001;44:S1–S12. doi: 10.1067/mjd.2001.109815. [DOI] [PubMed] [Google Scholar]
- 4.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blume-Peytavi U, Wahn U. Optimizing the treatment of atopic dermatitis in children: a review of the benefit/risk ratio of methylprednisolone aceponate. J Eur Acad Dermatol Venereol. 2011;25:508–515. doi: 10.1111/j.1468-3083.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Jung KE, Lee YB, Kim JE, Kim HS, Lee KH, et al. Use of emollients in atopic dermatitis: a questionnaire survey study. Ann Dermatol. 2014;26:528–531. doi: 10.5021/ad.2014.26.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. doi: 10.1016/j.jaad.2005.01.010. quiz 16-18. [DOI] [PubMed] [Google Scholar]
- 8.Choi EJ, Oh HM, Na BR, Ramesh TP, Lee HJ, Choi CS, et al. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm Res. 2008;25:1355–1364. doi: 10.1007/s11095-008-9531-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Lee M, Kim SH. Eupatilin inhibits H(2)O(2)-induced apoptotic cell death through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Food Chem Toxicol. 2008;46:2865–2870. doi: 10.1016/j.fct.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Choi EJ, Oh HM, Wee H, Choi CS, Choi SC, Kim KH, et al. Eupatilin exhibits a novel anti-tumor activity through the induction of cell cycle arrest and differentiation of gastric carcinoma AGS cells. Differentiation. 2009;77:412–423. doi: 10.1016/j.diff.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, et al. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol. 2007;7:1678–1684. doi: 10.1016/j.intimp.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, Kwon EY, Lee YS, Kim WB, Ro JY. Eupatilin blocks mediator release via tyrosine kinase inhibition in activated guinea pig lung mast cells. J Toxicol Environ Health A. 2005;68:2063–2080. doi: 10.1080/15287390500177024. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii Y, Takeuchi H, Sakuma S, Sengoku T, Takakura S. Characterization of a 2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats. Skin Pharmacol Physiol. 2009;22:240–247. doi: 10.1159/000235551. [DOI] [PubMed] [Google Scholar]
- 15.Jung KE, Lee YJ, Ryu YH, Kim JE, Kim HS, Kim BJ, et al. Effects of topically applied rapamycin and mycophenolic acid on TNCB-induced atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2015;26:432–438. doi: 10.1016/j.intimp.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Kim Y, Yi H, Jung H, Rim YA, Park N, et al. Eupatilin ameliorates collagen induced arthritis. J Korean Med Sci. 2015;30:233–239. doi: 10.3346/jkms.2015.30.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol. 1995;105:749–755. doi: 10.1111/1523-1747.ep12325538. [DOI] [PubMed] [Google Scholar]
- 19.Leonard WJ. TSLP: finally in the limelight. Nat Immunol. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- 20.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 21.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006;118:178–189. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Bao L, Alexander JB, Shi VY, Mohan GC, Chan LS. Interleukin-4 up-regulation of epidermal interleukin-19 expression in keratinocytes involves the binding of signal transducer and activator of transcription 6 (Stat6) to the imperfect Stat6 sites. Immunology. 2014;143:601–608. doi: 10.1111/imm.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 25.Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715–1721. doi: 10.1016/S0140-6736(97)12082-7. [DOI] [PubMed] [Google Scholar]