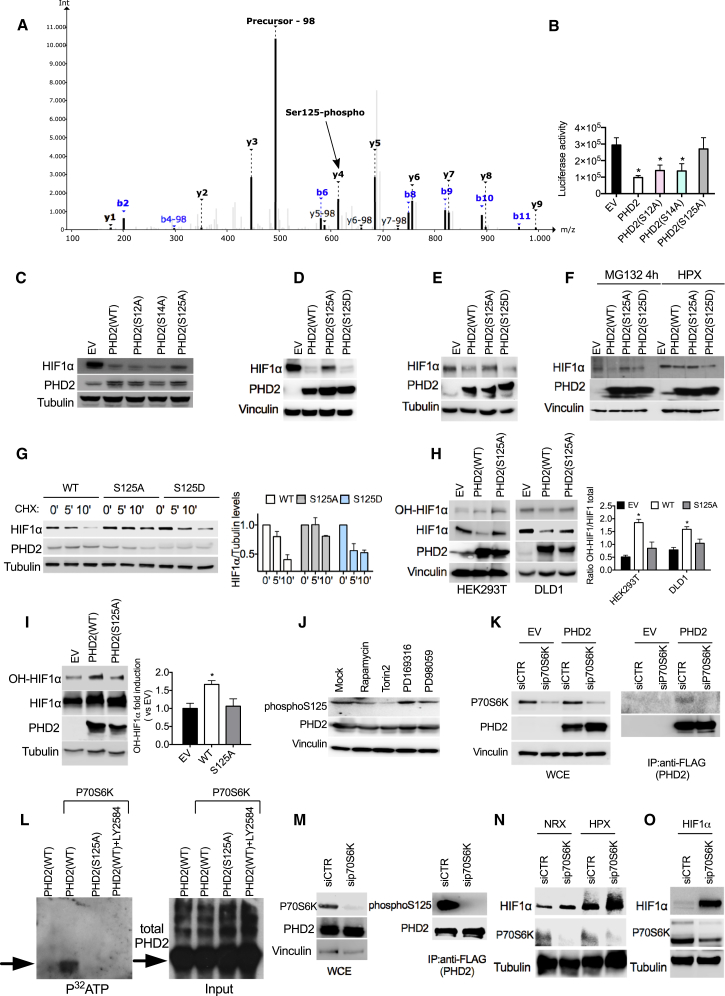
Figure 1.
Phosphorylation of S125 by P70S6K Is Important to Control HIF1α Levels
(A) MS/MS spectrum of NH2-AKPPADPAAAAS < p > PCR-COOH (S < p > indicates phosphoserine). Nine of 15 amino acids, including the phosphorylated serine, are covered by a ladder of y-type fragment ions. The neutral loss peak (−98 Da) characteristic for phosphorylated peptides is the most intense peak of the spectrum at 492.9 mass-to-charge ratio (m/z).
(B) Luciferase activity of HEK293T cells stably overexpressing an oxygen-dependent degradable luciferase (Luc-ODDD) transfected with plasmids carrying wild-type (WT) or mutant PHD2 (S12A, S14A, and S125A) or an empty vector (EV) as a control.
(C) Western blot (WB) of HEK293T cells transfected with HIF1α, alone (EV) or in combination with WT or mutant PHD2 (S12A, S14A, and S125A).
(D) WB of HEK293T cells transfected with HIF1α, alone (EV) or in combination with PHD2WT, PHD2S125A, or PHD2S125D.
(E) WB of DLD1 cells transfected with PHD2WT, PHD2S125A, and PHD2S125D and exposed to hypoxia for 4 hr.
(F) HT29 cells were transfected with an EV or with PHD2WT, PHD2S125A, and PHD2S125D. After 16 hr, cells were treated with the proteasome inhibitor MG132 or exposed to hypoxic conditions for 4 hr, and whole cell extracts (WCEs) were analyzed by WB.
(G) DLD1 cells were transfected with PHD2WT, PHD2S125A, and PHD2S125D. After 16 hr, cells were treated with cycloheximide for the indicated time, and WCEs were analyzed by WB.
(H) HEK293T or DLD1 cells were transfected with HIF1α, alone (EV) or in combination with PHD2WT or PHD2S125A. Hydroxylation of HIF1α was detected by using antibody against OH-HIF1α.
(I) WB of DLD1 cells transfected with PHD2WT, PHD2S125A, and PHD2S125D and treated with MG132 (10 μM) for 8 hr.
(J) WB of HEK293T cells transfected with PHD2 treated with 1 μM Rapamycin, 200 nM Torin2, 10 μM PD169316, and 10 μM PD19058 for 16 hr.
(K) DLD1 cells were transfected with either EV or FLAG-PHD2 and with a control (siCTR) or a small interfering RNA (siRNA) targeting P70S6K (siP70S6K). After 24 hr, cells were lysed, and WCEs were analyzed by WB (left). 1 mg of EV or FLAG-PHD2 lysate was immunoprecipitated using anti-FLAG M2 beads to detect endogenous P70S6K kinase (right).
(L) Purified FLAG-PHD2 and PHD2 S125 proteins were phosphorylated with active P70S6K kinase in the presence of [32P]-γ-ATP for 20 min at 30°C. The specific P70S6K inhibitor LY2584702 was used to a final concentration of 50 μM. Proteins were separated by 10% SDS-PAGE, and incorporated radioactivity was detected by autoradiography (left). A WB of PHD2 input was used as a loading control (right).
(M) HEK293T were transfected with FLAG-PHD2 and silenced with siP70S6K or siCTR. Then cells were lysed, and WCEs were analyzed by WB. 1 mg of EV or FLAG-PHD2 lysate was immunoprecipitated using anti-FLAG M2 beads to detect endogenous phosphorylated PHD2 (right).
(N) WB of DLD1 cells transfected with siCTR or siP70S6K and exposed to hypoxia for 4 hr.
(O) WB of HEK293T cells overexpressing HIF1α and silenced with siP70S6K or siCTR.
All WBs were repeated three times on independent biological replicates. ∗p < 0.05 versus EV in (B) and versus all other conditions in (H) and (I). Graphs show mean ± SEM. See also Figure S1.