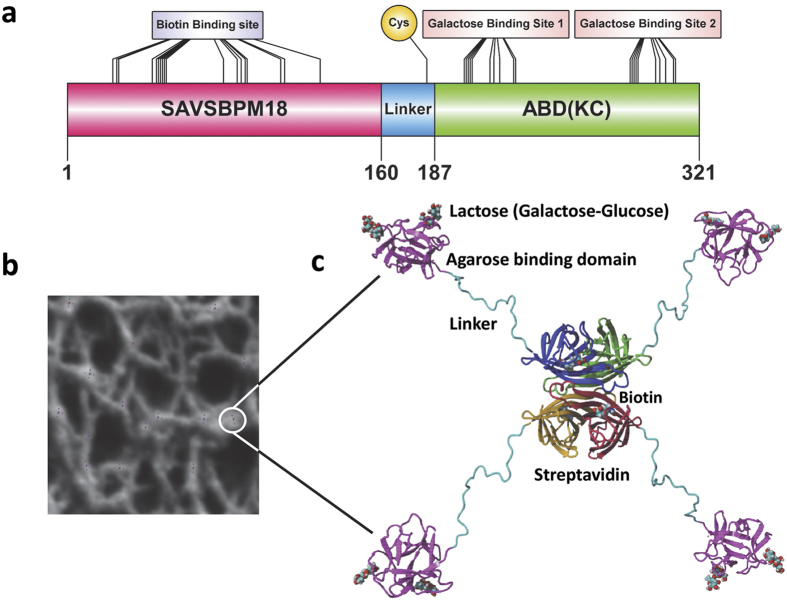
Figure 2. A diagram showing the primary and the modelled structures of the SAVSBPM18-L-ABD(KC) fusion and its immobilization to Sepharose 6B-CL.
(a) Schematic organization of SAVSBPM18-L-ABD(KC). Biotin binding site in streptavidin, cysteine-183 in the linker region and galactose binding sites in the agarose binding domain are shown. Galactose binding sites 1 and 2 correspond to the α and γ subdomains discussed in the text. (b) Binding of SAVSBPM18-L-ABD(KC) to agarose. The structure of SAVSBPM18-L-ABD(KC) is modelled. The diameter of the fusion in this model is 4 nm. In the agarose beads, many agarose strands assemble to form macromolecular strand-like structures with an average diameter in the range of 40–60 nm31. Many streptavidin fusions can bind to these structures located on the bead surface. The bound fusions are schematically shown in a larger size (i.e. not in proportion to agarose strands) for the purpose of illustration. One of the bound molecules is marked by a white circle. (c) Magnification of a surface bound streptavidin-agarose binding domain fusion. The four streptavidin subunits are colored red, brown, green and blue, respectively. Each subunit has a bound biotin. The agarose binding domains are shown in pink. Each domain interacts with two galactose residues present on the Sepharose bead surface. The galactose in complex with ABD is shown in the form of lactose (Galactose-Glucose). The linker region is shown in cyan.