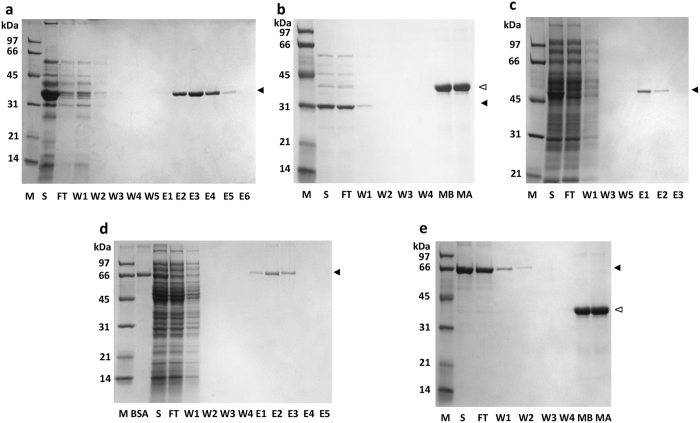
Figure 5. Purification of SBP-tagged or biotinylated proteins using SAVSBPM18-L-ABD(KC) Sepharose column.
(a) Purification of β-lactamase-SBP from the culture supernatant of B. subtilis. Arrowhead marks the position of β-lactamase-SBP. (b) Inability of β-lactamase (no SBP tag) from the B. subtilis culture supernatant to bind to SAVSBPM18-L-ABD(KC) Sepharose column. MB, boiled matrix (30 μl) from the affinity column before loading of β-lactamase (no SBP tag); MA, boiled matrix (30 μl) from the affinity column after sample loading and washing of the column. Solid arrowhead: β–lactamase; empty arrowhead: SAVSBPM18-L-ABD(KC). (c) Purification of biotinylated MBP-AviTag (one biotin per protein) from a crude sample containing intracellular E. coli cell extract. Pure biotinylated MBP-AviTag was mixed with the soluble fraction of E. coli to constitute the crude sample (S, second lane). Arrowhead marks the position of biotinylated MBP-AviTag. (d) Purification of biotinylated BSA (12 biotins per protein) from a crude sample containing intracellular E. coli cell extract. Pure biotinylated BSA was mixed with the soluble fraction of E. coli to constitute the crude sample (S, third lane). Arrowhead marks the position of biotinylated BSA. (e) Inability of non-biotinylated BSA to bind to SAVSBPM18-L-ABD(KC) Sepharose column. MB, boiled matrix (30 μl) from the affinity column before loading of non-biotinylated BSA; MA, boiled matrix (30 μl) from the affinity column after sample loading and washing of the column. Solid and empty arrowheads mark the positions of BSA and SAVSBPM18-L-ABD(KC), respectively. M, molecular weight markers; S, crude sample for all panels except panel e which has the pure non-biotinylated BSA as the sample; FT, flow-through fraction; W, wash fractions; E, elution fractions.