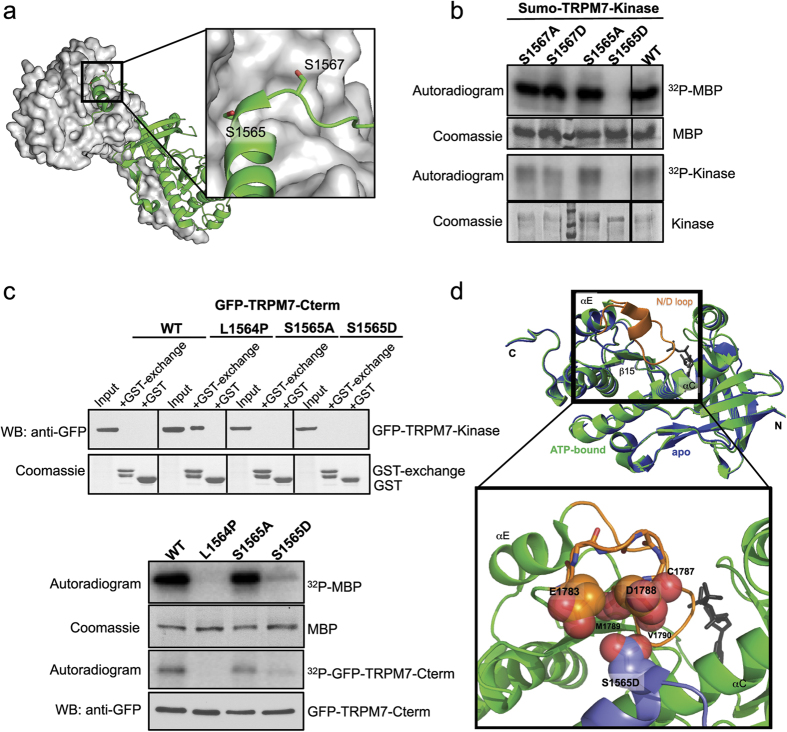
Figure 8. S1565 in the TRPM7 kinase dimerization exchange segment regulates catalytic activity.
(a) The structure of a dimerized TRPM7 kinase depicts the N-terminal exchange segment of one kinase monomer (ribbon diagram in green) interacting with another one (space-filled surface in gray). The expanded view on the right highlights the location of S1565 and S1567 on the exchange domain of one kinase monomer (side-chains shown as sticks). (b) An in vitro kinase assay assesses the catalytic activities of Sumo-TRPM7-Kinase WT and mutants carrying S1565A, S1565D, S1567A, and S1567D substitutions. The kinase assay was performed at 30 °C for 2 min. (c) GFP-TRPM7-Cterm containing WT kinase or mutants harboring the L1564P, S1565A, and S1565D substitutions were expressed in HEK-293T cells. Proteins in the lysates were affinity purified by GST-tagged mTRPM7 exchange segment (a.a. 1548–1576) (GST-exchange) bound to glutathione agarose. The affinity purified samples were resolved by SDS-PAGE and Coomassie blue staining. GFP-TRPM7-Cterm proteins were analyzed by immunoblotting with an anti-GFP antibody (top). An in vitro kinase assay was carried out to validate the catalytic activity of GFP-TRPM7-Cterm WT and mutant kinases. The kinase reaction was performed at 30 °C for 20 min. The presence of purified kinases was validated by western blotting using an anti-GFP antibody (bottom). (d) Overlaying image of the TRPM7 kinase in the ATP-bound state (PDB: 1IA9, green) and the apo-state (PDB: 1IAJ, blue). Orange color highlights the flexible N/D loop (a.a. 1783–1798) that is missing in the apo-state. The AMPPNP is shown as gray sticks. The zoomed panel at the bottom shows the flexible N/D loop (orange) of one kinase monomer superimposed with the exchange segment of another monomer containing the S1565D mutation (purple). Side chain carboxylates of D1565, E1783, and D1788, and backbone carbonyls of C1787, M1789, and V1790 are shown as red spheres.