Figure 1.
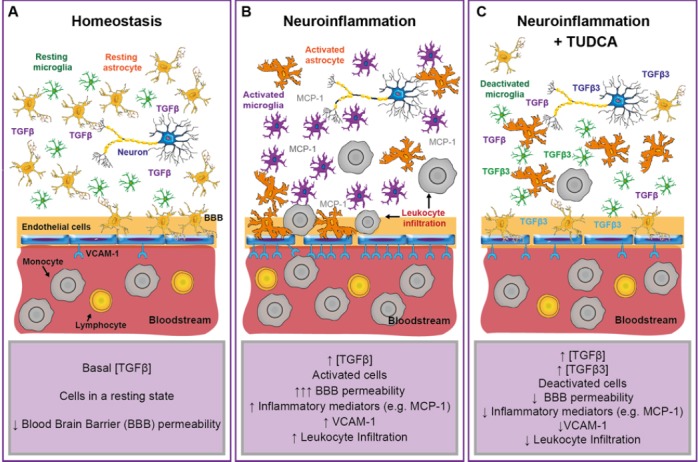
Proposed model for the effect of tauroursodeoxycholic acid (TUDCA) in the neuroinflammatory process.
(A) Under homeostatic conditions, the blood-brain barrier (BBB) restricts the entry of circulating blood cells into the central nervous system (CNS) parenchyma. The low permeability to circulating blood substances and leukocytes and the basal concentration of anti-inflammatory mediators (such as transforming growth factor β (TGFβ)) in the CNS allow a resting-homeostatic state in CNS resident cells. (B) CNS neuroinflammation drives the activation of resident glial cells (such as microglia and astrocytes) and the secretion of inflammatory mediators (e.g., monocyte chemoattractant protein 1 (MCP-1)) that promote the infiltration of peripheral leukocytes into the injured parenchyma. Endothelial cells increase the expression of surface adhesion molecules (e.g., vascular cell adhesion molecule 1 (VCAM-1)). Both, reactive astrocytes and activated endothelium, increase the permeability of the BBB. Circulating leukocytes adhere to the activated endothelium and infiltrate into the CNS, perpetuating the inflammatory response by a direct interaction with neurons and glial cells. During early inflammation, the activation of matrix metalloproteases releases TGFβ from the extracellular matrix, increasing the activation of the TGFβ pathway and counterbalancing the pro-inflammatory response. (C) Under inflammatory conditions, TUDCA treatment increases the activation of the TGFβ pathway and the expression of TGFβ3 in neurons, microglia and endothelial cells. The activation of TGFβ pathway drives the deactivation of glial cells and the endothelium, leading to an anti-inflammatory microenvironment with reduced expression of vascular adhesion molecules (e.g., VCAM-1) and inflammatory mediators (e.g., MCP-1), and therefore to a reduced BBB permeability and leukocyte infiltration.
