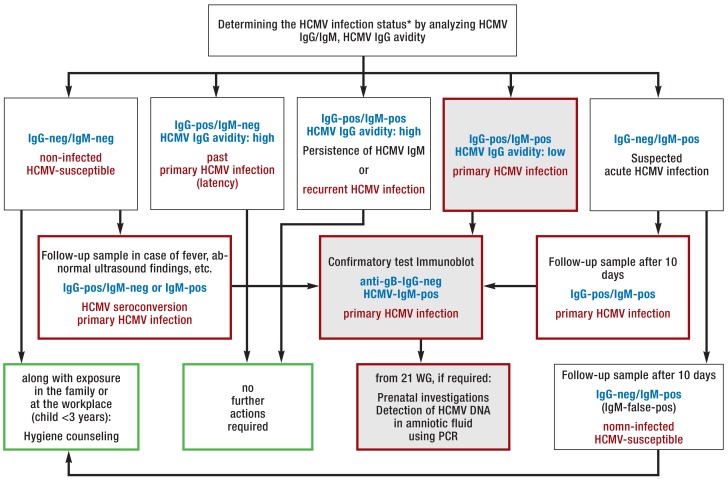
Figure 2.
Laboratory diagnostic procedure (from [e11]: Courtesy of the German Association for the Control of Virus Diseases (Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten) and the German Society for Virology) (Deutsche Gesellschaft für Virologie) to determine the human cytomegalovirus (HCMV) infection status in pregnant women with suspected primary HCMV infection, combined with possible actions.
Blue font: Result constellation; red font: interpretation; green border: actions; red border: further investigations required; gray background: constellation of results typical for primary HCMV infection.
* Note: The HCMV serostatus is determined at the start of pregnancy (HCMV screening), solely based on HCMV immunoglobulin (Ig)G testing. For more detailed information refer to the S2K guideline of the Association of the Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) on the laboratory testing recommended to assess viral infections relevant in pregnancy (e11).
DNA, deoxyribonucleic acid; false-pos, false positive; neg, negative; PCR, polymerase chain reaction; pos, positive; WG, weeks’ gestation