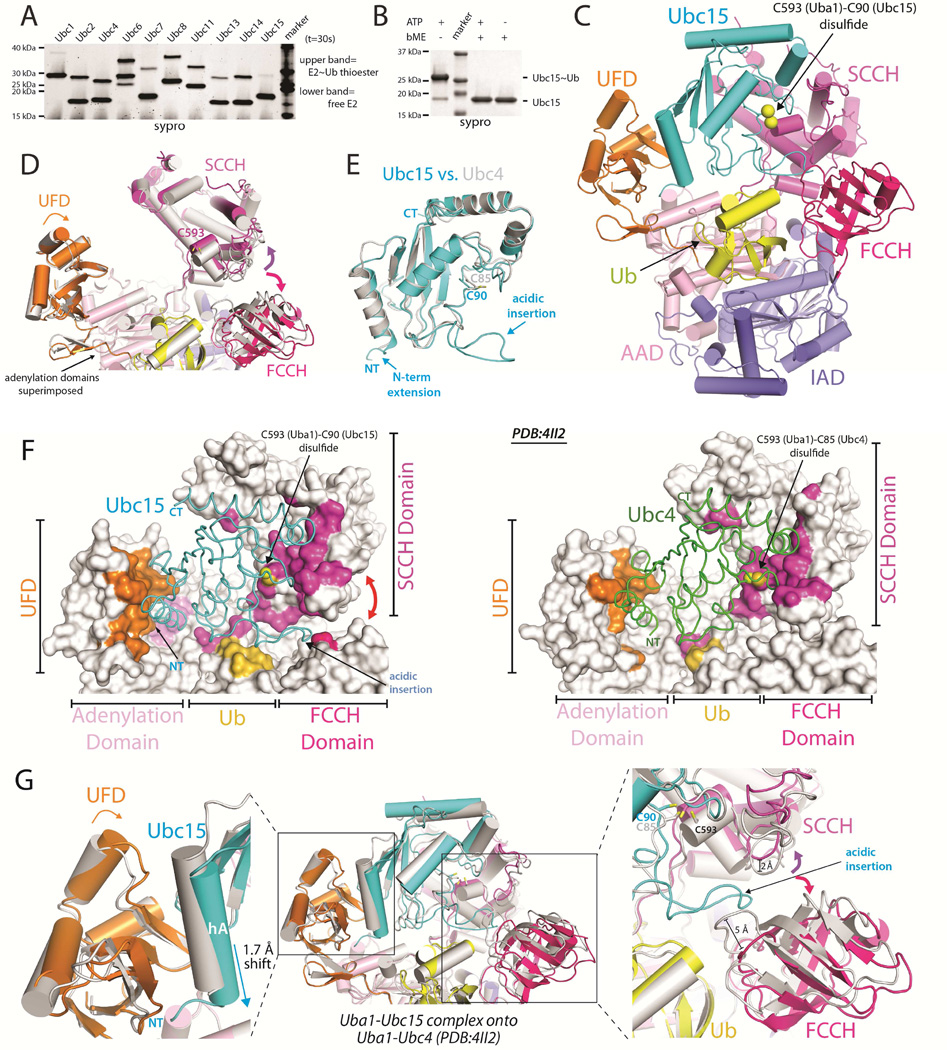
Figure 1. Uba1-Ubc15/Ub structure reveals a distinct Ub E1 binding mode.
(A) E1-E2 Ub thioester transfer assays for the indicated Uba1-E2 pairs.
(B) Uba1-Ubc15 thioester transfer assay under endpoint conditions, prepared in the presence and absence of reducing agent.
(C) Cartoon of the Uba1-Ubc15/Ub complex with Uba1 domains color-coded and labeled.
(D) Uba1 from the Uba1-Ubc15 structure is colored as in C and Uba1 from the Uba1-Ubc4 structure (PDB: 4II2) is colored gray. Uba1 adenylation domains superimposed (RMSD=0.207 Å). Domain rotations indicated with arrows.
(E) The UBC domains of Ubc15 (cyan) and Ubc4 (gray) were superimposed and the structures are shown as ribbons.
(F) Uba1-Ubc15 (left) and Uba1-Ubc4 (right) structures with Uba1 atoms contacting E2 colored by domain, as in C.
(G) Ubc15 and Ubc4 Uba1 binding modes. The adenlyation domains were superimposed and colored as in D and the E2s are shown as cartoons and colored as in E.