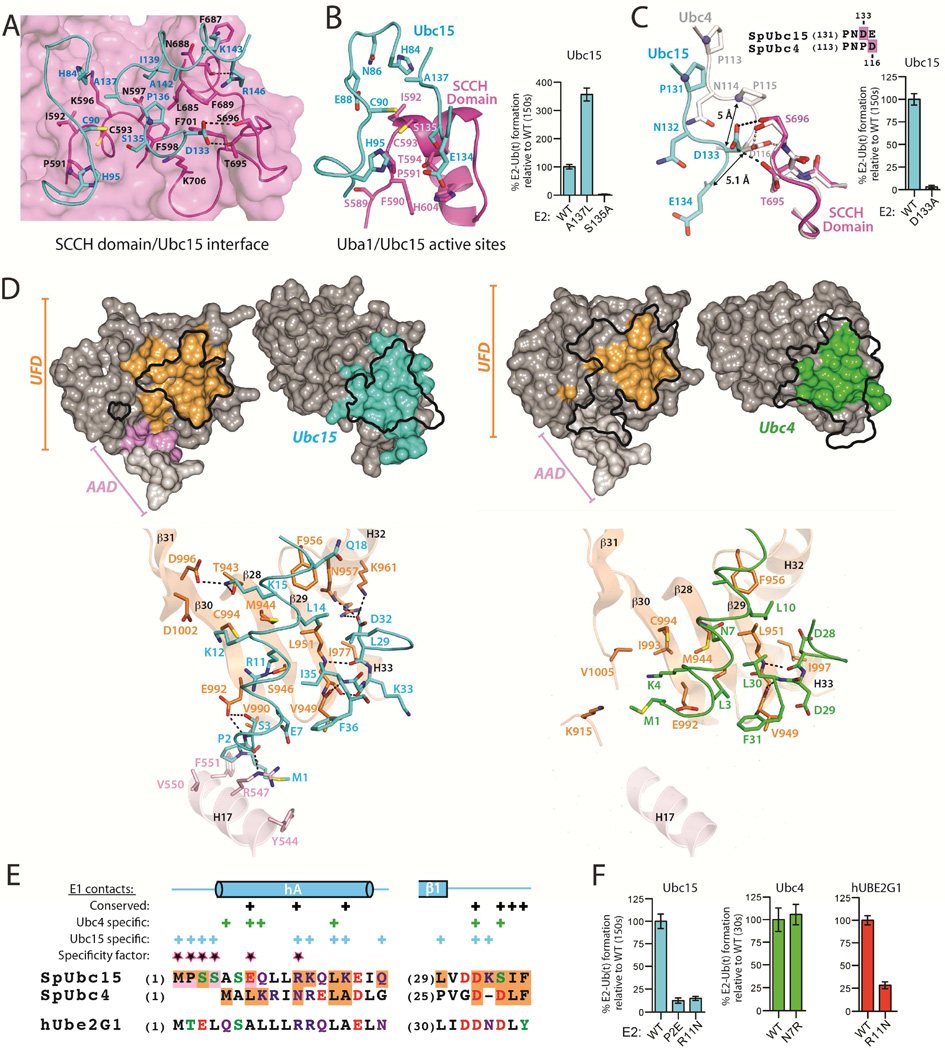
Figure 3. The Ubc15 mode of Uba1 binding.
(A) Uba1-Ubc15 structure colored as in Figure 1C with residues involved in key intermolecular contacts shown as sticks.
(B) Close-up view of the environment surrounding the Uba1 and Ubc15 active site cysteines (left). E1-E2 thioester transfer assays of the indicated proteins (right).
(C) Selected equivalent E2 residues that occupy different locations at the E2/Cys domain interface indicated by a black arrow. SCCH domains superimposed (left). E1-E2 thioester transfer assays of the indicated proteins (right).
(D) Surface representation of the Ubc15/UFD (left) and Ubc4/UFD (PDB: 4II2; right) interfaces in open book representation with residues involved in intermolecular contacts colored as in Figure 1. Outlines of the Ubc15/UFD and Ubc4/UFD interfaces are mapped onto the opposing structures as a black outline. Ribbon representations of the Ubc15/UFD and Ubc4/UFD interfaces with residues involved in key intermolecular contacts shown as sticks (bottom).
(E) Structure-based sequence alignment highlighting regions at the E2/UFD interface. Residues contacting the UFD and AAD are shaded orange and pink, respectively. Residues involved in unique contacts are labeled as ‘specific contacts’ above the alignment, those involved in equivalent contacts are labeled as ‘conserved’. Residues that our structure-function studies show are important in determining the Ubc15 and Ubc4 modes of binding are termed ‘specificity factors’. Coloring: acidic residues (red), basic residues (blue), Ser/Thr/Tyr (green), Gln/Asn (purple) and all others black.
(F) E1-E2 thioester transfer assays of the indicated proteins.
See also Figure S3.