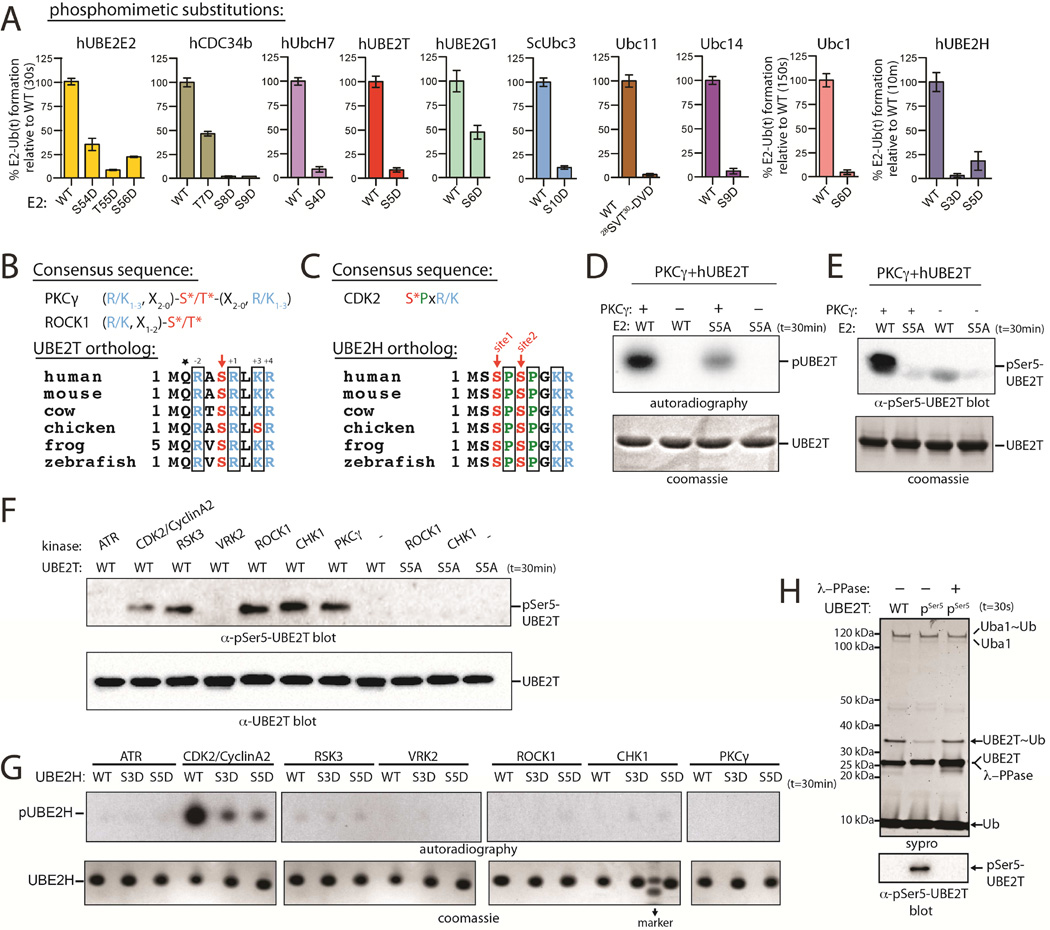
Figure 5. Phosphomimetic substitution or phosphorylation of N-terminal Ub E2 residues diminishes Ub E1-E2 thioester transfer activity.
(A) E1-E2 thioester transfer assays of the indicated proteins.
(B) PKCγ and ROCK1 consensus sequences (top). ‘X’ is any residue. Sequence alignment of UBE2T orthologs (bottom). Conserved residues consistent with the PKC consensus are in black boxes.
(C) CDK2 consensus sequence (top). Sequence alignment of UBE2H orthologs (bottom). Conserved residues consistent with the CDK2 consensus are in black boxes.
(D) In vitro radiometric PKCγ kinase assays with the indicated Ub E2 substrate (top). Coomassie stained loading control (bottom).
(E) In vitro gel-based PKCγ kinase assay. Samples were probed by western blot with pSer5-specific hUBE2T antibody (top). Coomassie stained loading control (bottom).
(F) In vitro phosphorylation of hUBE2T with the indicated kinase (top).
(G) In vitro phosphorylation of hUBE2H using the in vitro radiometric assay as in D (top).
(H) E1-E2 thioester transfer assays of WT and pSer5 UBE2T in the presence and absence of lambda phosphatase (top). Coomassie stained loading control (bottom).
See also Figure S5.