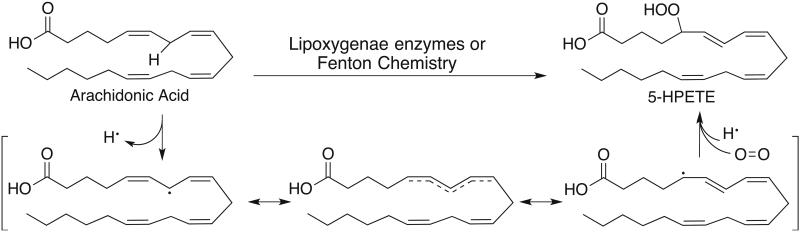
Figure 1.
General mechanism for peroxidation of PUFAs. A hydrogen atom at a bisallylic position is remove using either a radical or a redox active metal to generate a resonance stabilized carbon centered radical. The double bonds of the acid isomerize to form the more thermodynamically stable conjugated diene prior to reacting with molecular oxygen.