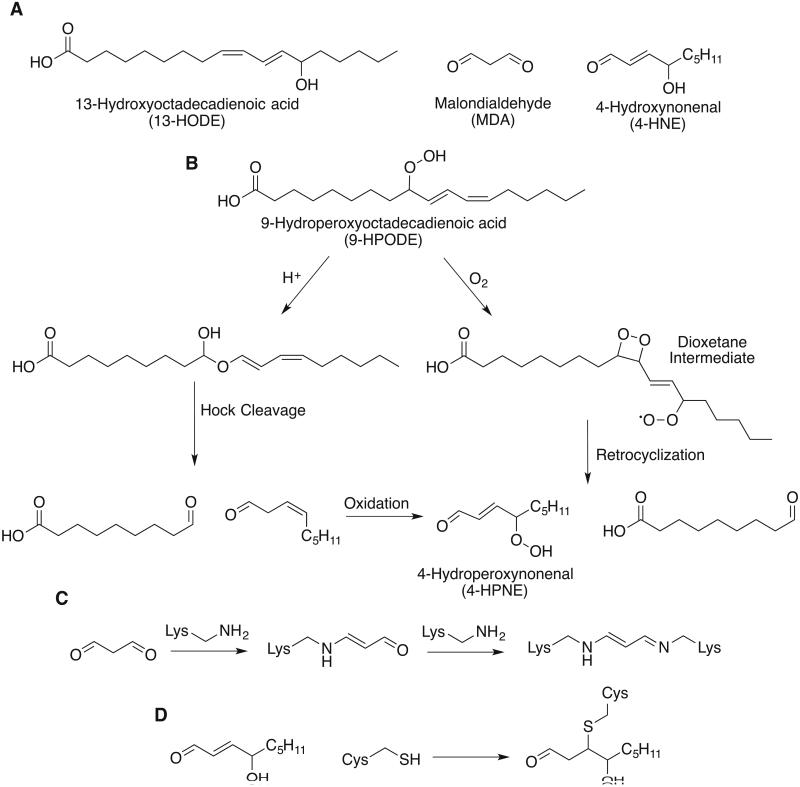
Figure 3.
Lipid peroxide degradation. (A) Structures of common lipid peroxide degradation products. (B) Two mechanistic hypotheses to explain the formation of 4-HNE via a peroxide intermediate 4-HPNE (C) Malondialdehyde can form Schiff bases with primary amines. In the first condensation, the resulting imine tautomerizes to the enamine before condensing with a second primary amine. (D) 4-hydroxynonenal is a Michael receptor that reacts with nucleophilic side chains such as cysteine.