Supplemental Digital Content is available in the text
Keywords: solifenacin, SRS, tamsulosin, terazosin, tolterodine, ureteric stent-related discomfort
Abstract
Background:
As a monotherpay, a-blockers and anti-muscarinics are both efficacy for ureteral stent-related symptoms (SRS). The aim of the study was to systematically evaluate their efficacy of a combination therapy for SRS.
Methods:
Relevant studies investigating α-blockers and/or anti-muscarinics for SRS were identified though searching online databases including PubMed, EMBASE, Cochrane Library, and other sources up to March 2016. The RevMan software was used for data analysis, and senesitivity analysis and inverted funnel plot were also adopted.
Results:
Seven randomized controlled trials (RCTs) and 1 prospective controlled trial including 545 patients were selected. Compared with α-blockers, the combination group achieved significant improvements in total International Prostate Symptom Score (IPSS) [–3.93 (2.89, 4.96), P < 0.00001], obstructive subscore [–1.29 (0.68, 1.89), P < 0.0001], irritative subscore [–2.93 (2.18, 3.68), P < 0.00001], and quality of life score [–0.99 (0.42, 1.55), P < 0.001]. Compared with antimuscarinics, there were also significant differences in total IPSS [–3.49 (2.43, 4.55), P < 0.00001], obstructive subscore [–1.40 (0.78, 2.01), P < 0.00001], irritative subscore [–2.10 (1.30, 2.90), P < 0.00001], and quality of life score [–1.18 (0.58, 1.80), P < 0.001] in favor of combination group. No significant difference was found in the visual analog pain score and the urinary symptoms score in Ureteral Stent Symptom Questionnaire (USSQ). No significant difference in complications was found.
Conclusions:
Current analysis shows significant advantages of combination therapy compared with monotherapy of α-blockers or antimuscarinics alone mainly based on IPSS. More RCTs adopting validated USSQ as outcome measures are warranted to support the finding.
1. Introduction
Ureteral stents are usually applied in urolithiasis, obstructive pyelonephritis, and after some endoscopic procedures in the case of ureteral edema and ureter perforation.[1,2] Though dilate urinary tract, they assist in kinds of aspects including ureter stone passage, renal pelvic pressure reduction, obstruction prevention, and injury recovery acceleration.[3] However, because of their invasion to all of renal, ureter, and bladder, discomforts and morbidities were also unavoidable to be related with stents insertion.
The reported morbidities mainly included lower urinary tract symptoms, pain, hematuria, and infections. A series of symptoms were demonstrated to be stent-related, and the stent-related symptoms (SRS) affected over 80% of the patients,[4] and sometimes SRS also had obvious influence to general health status and work performance. To reduce the incidence of SRS, the material and size of stents were primary considered and adjusted. After that, some drugs that are effective in the treatment of benign prostatic hyperplasia-related symptoms are also attempted to be administrated in clinic.[5] Among kinds of drugs, α-blockers and antimuscarinics were mostly adopted, and both of them were demonstrated to be helpful to relieve the severity of SRS in the patients.[6,7]
Although comparative efficacy of the 2 drugs for SRS was still unclear due to different pharmacological effects between α-blockers and antimuscarinics, some studies immediately supposed an advantage of combination therapy of both of them compared monotherapy of either of them.[8–15] Combined administration may finally enhance the therapeutic effects; however, results of previous studies with limited sample sizes were not completely consistent, and thus certain conclusions were absent. As ureteral stents were commonly used in urology even as a routine procedure after the ureter surgery, a more effective treatment method than before for SRS would be very practical for urologists. Therefore, we gathered all relevant prospective controlled studies to systematically evaluate the efficacy of combination therapy compared with monotherapy of a-blockers and antimuscarinics for SRS.
2. Materials and methods
2.1. Search strategy
A comprehensive literature search was performed in PubMed, EMBASE, the Cochrane Library, and other sources such as clinical trial register centers up to 16 March 2016. Search terms were as followings: (alpha-blocker OR α-blocker OR tamsulosin) AND (antimuscarinic OR tolterodine OR solifenacin) AND (ureteral stent-related symptoms OR ureteric stent-related discomfort OR SRS). Detailed search strategy in PubMed can be found in Supplementary materials. At the same time, references and related articles of potential clinical studies and reviews were also manually checked. Language was limited to English.
2.2. Inclusion criteria
Published studies investigated the efficacy of combination therapy of α-blockers and antimuscarinics versus monotherapy of α-blockers or antimuscarinics were considered. Study designs were limited to prospective clinical controlled trials, whereas reviews, case series, retrospective studies, and animal studies were excluded. Participants were patients undergoing a double-J ureter stent placement after urinary surgery, due to ureteral stones and/or other diseases. Interventions were α-blockers, antimuscarinics, and anesthetics on demand. Patients in the control group (monotherapy group) received either α-blockers or antimuscarinics, whereas patients in the treatment group (combination group) received both of them. Specific drugs administrated in the included trials were tamsulosin, terazosin, tolterodine, and solifenacin.
Outcome measures used to present the efficacy and safety of the therapy as follows. Primary outcomes included International Prostate Symptom Score (IPSS). Secondary outcomes included Ureteral Stent Symptom Questionnaire (USSQ), quality of life (QoL) score, visual analog pain score (VAS), and complications.
2.3. Data abstraction
According to the inclusion criteria, searched citations were primary screened by the titles and abstracts to exclude nonrelevant studies. And potential citations for inclusion were further confirmed by full-texts evaluation. After that, the basic characteristics, methodological quality items, and data of outcome measures were abstracted in predesigned tables. The process was completed by 2 reviewers and cross-checked independently.
2.4. Quality assessment
The quality of included studies was assessed by using the methods recommended by the Cochrane Handbook, which included 6 domains: randomization, allocation concealment, blinding of participant and outcome measurement, incomplete outcomes, selective reporting, and other bias.[16] It reflected the risk of bias possibly located in the process of selection, performance, detection, follow-up, reporting, and others.
2.5. Statistical analysis
The RevMan software (5.3 version, recommended by the Cochrane Collaboration) was used to analyze the data of outcomes. The clinical heterogeneity was first handled by subgroup analyzes, and then statistical heterogeneity was tested by the Q statistic and the chi-I2 statistic, which was presented as the value of I2. Significant heterogeneity was considered when I2 >50%, and a random-effects was used. Otherwise, a fixed-effect model was used. Mean difference (MD) with 95% confidence interval (CI) was used to reflect the overall effect size for continuous variances, and risk ratio (RR) with 95%CI was used for dichotomous variances. For continuous outcomes, changes from the level of baseline (mean1+SD1) to the level of outcome measured time (mean2+SD2) were first calculated using the formula as previously reported in the treatment group and in the control group, respectively:[17] {mean = mean2 − mean1, standard deviation (SD) = SQRT (SD12 + SD22 − 2r × SD1 × SD2), r was define as 0.5}, and then the changes were then compared with each other. Difference between the 2 groups was considered significant when P < 0.05. Sensitivity analysis was performed though changing combined models and/or omitting study with high risks. The inverted funnel plot method was adopted to explore the risk of publication bias by visually judging the shape of the plots.
Current meta-analysis was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). It did not involve ethical issue.
3. Results
3.1. Characteristics of the included studies
Literature search yielded 167 citations, and a total of 8 trials[8–15] containing 269 cases in the monotherapy group and 276 cases in the combination group were finally included (Fig. 1). Among the included studies, 7 of them were designed as randomized controlled trials (RCTs) and 1 was prospective controlled trial. Baseline characteristics were presented in Table 1. As shown, the sex ratio and average age were comparable. Mainly primary disease was ureteral stones, and only 1 trial also included a small proportion of transitional cell carcinoma patients. The stent size was reported to be fixed in 4 trials,[8–10,14] and to be adjusted by weight and height in 2 trials,[11,15] and the other 2 trials[12,13] did not report detailed information. Average duration of stent insertion ranged from 8.7 days to 23.3 days across the trials. Administrated α-blockers included tamsulosin in 7 trials and terazosin in 1 trial,[10] and administrated antimuscarinics included tolterodine in 5 trials and solifenacin in 3 trials.[9,11,14]
Figure 1.
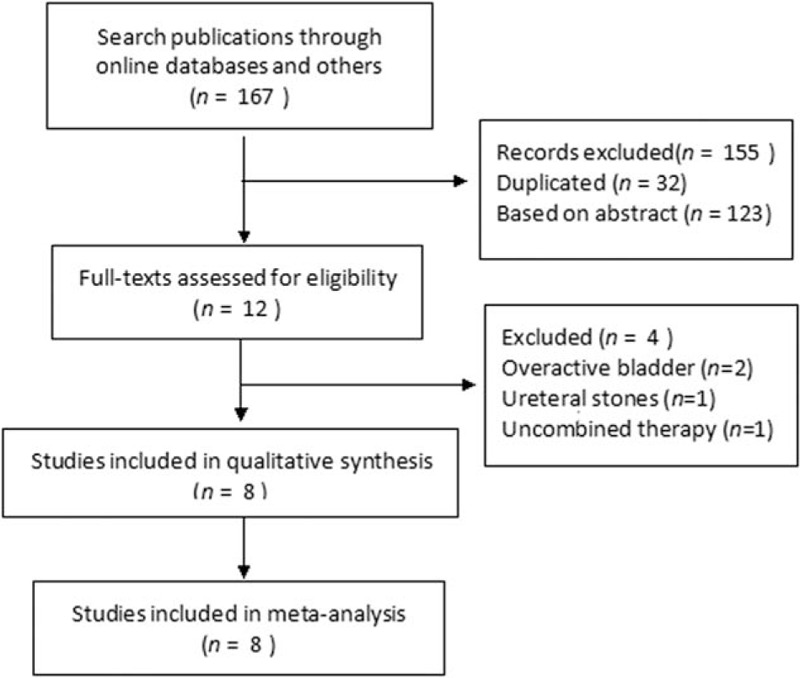
Flowchart of literature search.
Table 1.
Baseline characteristic of included trials.
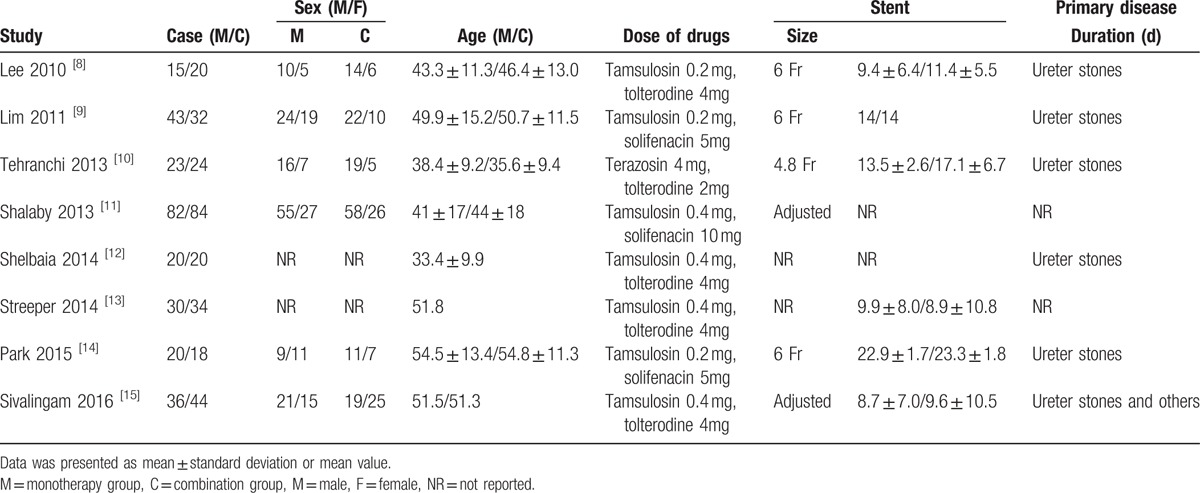
Quality assessment results showed that the overall quality of methodology was low to moderate. One study[9] had high risk and 2 studies[8,12] had unclear risk in randomization, and only 1 study[10] had low risk in allocation concealment and 4 studies[10,13–15] had low risk in blinding (Table 2).
Table 2.
Quality assessment of included studies.
3.2. International prostate symptom score (IPSS)
Meta-analysis results of IPSS score involving 471 patients.[8–11] Compared with α-blockers alone, combination therapy led to a significant reduction in the total IPSS score by a mean of 3.93 (95%CI, 2.89–4.96, P < 0.00001), the obstructive subscore by a mean of 1.29 (95%CI, 0.68–1.89, P < 0.0001), and the irritative subscore by a mean of 2.93 (95%CI, 2.18–3.68, P < 0.00001); as shown in Figs. 2 and 3.
Figure 2.
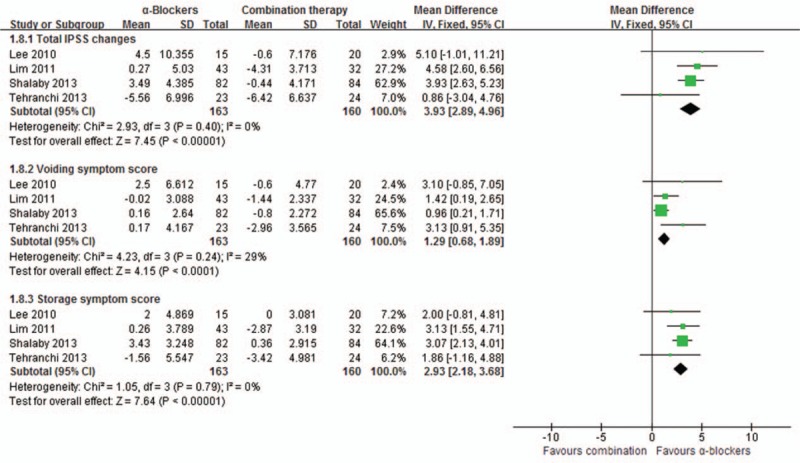
Meta-analysis of International Prostate Symptom Score of α-blockers versus combination.
Figure 3.
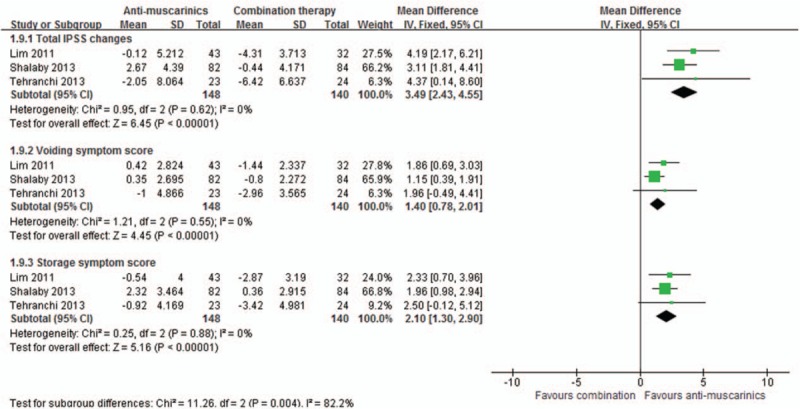
Meta-analysis of International Prostate Symptom Score of antimuscarinics versus combination.
Compared with antimuscarinics alone, combination therapy also achieved a significant reduction in the total IPSS score by a mean of 3.49 (95%CI, 2.43–4.55, P < 0.00001), the obstructive subscore by a mean of 1.40 (95%CI, 0.78–2.01, P < 0.00001), and the irritative subscore by a mean of 2.10 (95%CI, 1.30–2.90, P < 0.00001), as shown in Figs. 2 and 3.
3.3. Urinary symptoms score
Meta-analysis results of urinary symptoms score in USSQ involving 182 patients,[13–15] and a descriptive analysis was performed due to detailed data absent. All of the studies showed that there was no significant difference between the groups (P = 0.84, 0.16, 0.52, respectively), although each group achieved a significant improvement of urinary symptoms scores at outcome measured time compared with the baseline level.
3.4. Quality of life (QoL)
Meta-analysis results of 6 studies[8–11,14,15] including 609 cases reported QoL. Combination therapy significantly reduced the QoL score by a mean of 0.99 (95%CI, 0.42–1.55, P = 0.0007) compared with α-blockers alone, and also significantly reduced the score by a mean of 1.18 (95%CI, 0.58–1.80, P = 0.0002) compared with antimuscarinics alone, as shown in Fig. 4.
Figure 4.
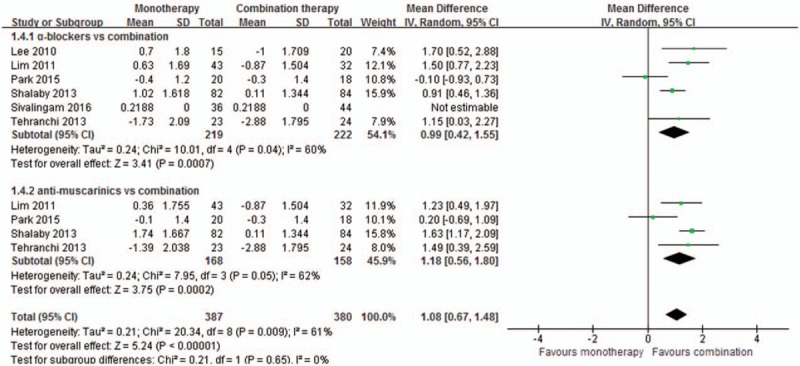
Meta-analysis of quality of life score changes between the groups.
3.5. Visual analog pain score (VAS)
Meta-analysis results of 4 studies[8–11] involving 471 patients showed that there was no significant difference between the combination group and the monotherapy group of either α-blocker (MD = 0.45, 95%CI [0.01, 0.90], P = 0.05) or antimuscarinic (MD = 0.63, 95%CI [–0.35, 1.62], P = 0.21), as shown in Fig. 5.
Figure 5.
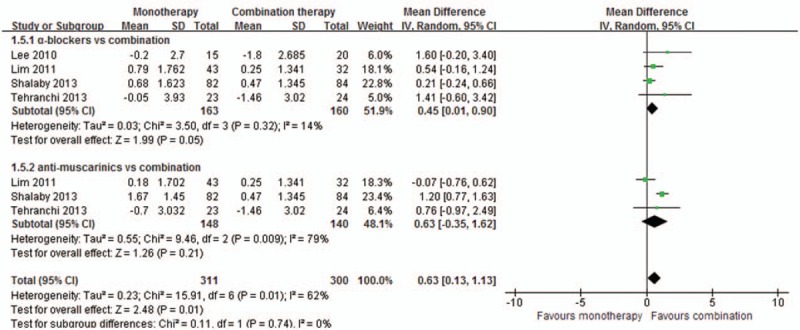
Meta-analysis of visual analog pain score changes between the groups.
3.6. Complications
Only 2 studies[8,14] reported the data of treatment-related complications, and the others stated that no related complication was found. Meta-analysis results demonstrated no significant difference between combination and monotherapy (RR = 0.84, 95%CI [0.25, 2.82], P = 0.78). The reported complications were mild, which included fatigue, dyspepsia, dizziness, constipation, vision blurred, and abdominal discomfort.
3.7. Sensitivity analysis
For outcomes measures combined in the fixed-effect model, though converting the combined model into the random-effect model, the changing trends were not altered in aspects of total IPSS, the obstructive subscore, the irritative subscore, and complications between combination therapy with α-blockers or antimuscarinics alone. For outcomes measures combined in the random-effect model, though omitting study with high risks,[9] the changing trends were not altered in aspects of QoL and VAS between combination therapy with antimuscarinics, while altered in outcomes of VAS between combination therapy with α-blockers (MD = 0.70, 95%CI [–0.26, 1.65], P = 0.15).
3.8. Publication bias
Inverted funnel plots indicated that risks of bias might exist in outcomes of obstructive subscore and VAS. However, low risks of bias may exist in outcomes of total IPSS score, irritative subscore, and QoL score (Supplementary materials).
4. Discussion
The use of α-blockers and antimuscarinics for SRS was mainly based on the similarity of lower urinary tract symptoms frequently happened in benign prostatic hyperplasia and overactive bladder.[7,18] α-Blockers was also adopted as first-line drugs in medical explosive therapy for ureteral stones, and it was reported to be able to maintain the baseline frequency of spontaneous contractility while reduce the persisted pressure in ureter.[19] For antimuscarinics, they can inhibit the activity of muscarinic receptor and the involuntary contraction of bladder.[20] As a stent affected both ureter and bladder, combined α-blocker and anti-muscarinic may be a very promising therapy for SRS.
Our meta-analysis included 8 studies and showed significant benefits of combination therapy than α-blockers or antimuscarinics alone for SRS. The results showed that the combination group was associated with significantly reduced total IPSS score, obstructive subscore, and irritative subscore. IPSS was actually a specific questionnaire for benign prostatic hyperplasia.[21] It included 2 aspects of obstructive subscore and irritative subscore, and reflected the severity of voiding and storage disfunction. For SRS patients, such symptoms may be mainly caused by the stimulation of distal curling of the stent in bladder, and thus, it was very important to adjust the length of stent according to patients’ height to avoid the distal curling located to much in the bladder or crossed the midline of the bladder.[8] However, after adjusting stent position in bladder, large parts of patients still suffered severe SRS. Previous studies have already demonstrated the positive effects and further compared the difference across kinds of α-blockers,[22] and it is clear that α-blockers would block the stimulation of stent to the area of bladder trigone, which plays crucial roles in its spontaneous activities.[23] Our meta-analysis suggested that additional antimuscarinics would further enhance the effects. Although concerns about the inhibition of antimuscarinics to detrusor muscle contraction were existed theoretically,[7] our data revealed that the obstructive subscore was not to be worsen but improved, and this may be determined by the dose of antimuscarinics used in the included studies. The stent inserted duration was enabled to be comparable in each study, and after that, all the data of outcome measures were primarily compared with their baseline level and the value of changes were calculated and compared between combination and monotherapy groups, which would be useful to eliminate the influence of baseline level of SRS severity across the studies.
Besides, Park et al[14] stated that difference located in assessment tools and outcome assessment timing, which was the main cause inducing conflicting results and conclusions. Current analysis highly agreed with the points, and multiple outcome measures were adopted. We adopted the urinary symptoms score in USSQ, which had been accepted by more and more studies in recent years.[24] However, only 3 studies involving 182 patients[13–15] were included in the descriptive analysis, all of them failed to find any significant difference between combination and monotherapy groups. USSQ was demonstrated to be a validated scale specifically for patients suffered SRS, focusing on not only urinary symptoms, but also a more comprehensive evaluation than IPSS including bodily pain, general health, work performance, sexual matter, and quality of life. As the anesthetics were used on demand without limitation, so it would partly affect the subscores of VAS pain scores. However, our analysis prompted a significant improved QoL in favor of combination therapy. Besides, a significant improvement in sexual matters was also proposed. Park et al[14] concerned that the dropout may influence the results in their study, and we noticed that the prolonged duration of stent may also be an important clinical factor, as some researchers found that longer duration induced higher tolerance.[25] And in the study of Streeper et al[13] and Sivalingam et al,[15] they both adopted an early release tolterodine, and the authors explained that the time frame may not have permitted tolterodine to reach the optimal therapeutic window, so it seemed that the kind of antimuscarinics would also significant influence clinical outcomes. Thus, more well-designed RCTs applying validated USSQ as primary outcome measures are need to better support the advantages of combination therapy.
For safety, combination therapy did not induce more incidence of complications than monotherapy, as all of the included drugs were essentially associated with very rare and mile events. Meanwhile, there was still some other problems, as it was reported that such mild complications as fatigue, dizziness, vision blurred, and abdominal discomfort may to some extent influence the compliance of the patients,[14,26] especially in the employed population.
The limitations of current study were as follows. (1) In methodological quality assessment, only 1 study properly performed allocation concealment, and 4 studies adopted blinding. Allocation concealment as an action to prevent the grouping information from being known by the investigators who had the sequence number, it can be realized by sealed envelopes or center-controlled system.[27] And blinding of participants and outcomes assessment would avoid subjective bias from participants and investigators, and can be achieved by placebo and other methods.[28] The risks existed in these items might to some extent overestimate the effect size. Future RCTs should pay more attentions in the study design to increase the reliability. (2) For outcomes, current analysis results mainly based on IPSS is similar but more comprehensive and reliable than a previous meta-analysis.[7] As mentioned above USSQ is superior to IPSS for patients suffered SRS, although 3 of the included studies adopted USSQ as outcome measure, limited to insufficient sample size and data presentation, only a descriptive analysis was conducted and the primary results focusing on other items besides urinary symptoms were really attracting. (3) All of the material, size, position, and duration of inserted stent have some influence to outcomes,[29] although they were comparable in each study, they might cause unavoidable heterogeneity across the studies. (4) Publication bias always existed and was largely unrecognized, which had been well addressed in previous studies.[30] After applying the methods of inverted funnel plots, high risk of publication bias may exist in outcomes of obstructive subscore and VAS, which might also have negative influence to the reliability of the results.
5. Conclusions
Current analysis shows significant advantages of combination therapy compared with monotherapy of either α-blockers or antimuscarinics mostly based on outcome measures in IPSS. More well-designed RCTs adopting validated USSQ as primary outcome measures are warranted to supporting the finding.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, IPSS = International Prostate Symptom Score, MD = mean difference, QoL = quality of life, RCTs = randomized controlled studies, RCTs = randomized controlled trials, RR = risk ratios, SD = standard deviation, SRS = stent-related symptoms, USSQ = Ureteral Stent Symptom Questionnaire, VAS = visual analog pain score.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Auge BK, Preminger GM. Ureteral stents and their use in endourology. Curr Opin Urol 2002;12:217–22. [DOI] [PubMed] [Google Scholar]
- [2].Althaus AB, Li K, Pattison E, et al. Rate of dislodgment of ureteral stents when using an extraction string after endoscopic urological surgery. J Urol 2015;193:2011–4. [DOI] [PubMed] [Google Scholar]
- [3].Clayman RV. The use of stents in contemporary urology. J Urol 2004;172:2106. [Google Scholar]
- [4].Joshi HB, Stainthorpe A, Macdonagh RP, et al. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol 2003;169:1065–9. [DOI] [PubMed] [Google Scholar]
- [5].Kuyumcuoglu U, Eryildirim B, Tuncer M, et al. Effectiveness of medical treatment in overcoming the ureteral double-J stent related symptoms. Can Urol Assoc J 2011;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang P, Hu WL, Cheng B, et al. α1-blockers for the reduction of ureteric stent-related symptoms: a systematic review and meta-analysis. Exp Ther Med 2016;11:660–8. [J]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou L, Cai X, Li H, et al. Effects of α-blockers, antimuscarinics, or combination therapy in relieving ureteral stent-related symptoms: a meta-analysis. J Endourol 2015;29:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee SJ, Yoo C, Oh CY, et al. Stent position is more important than α-blockers or anticholinergics for stent-related lower urinary tract symptoms after ureteroscopic ureterolithotomy: a prospective randomized study. Korean J Urol 2010;51:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lim KT, Kim YT, Lee TY, et al. Effects of tamsulosin, solifenacin, and combination therapy for the treatment of ureteral stent related discomforts [J]. Korean J Urol 2011;52:485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tehranchi A, Rezaei Y, Khalkhali H, et al. Effects of terazosin and tolterodine on ureteral stent related symptoms: A double-blind placebo-controlled randomized clinical trial. Int Braz J Urol 2013;39:832–40. [DOI] [PubMed] [Google Scholar]
- [11].Shalaby E, Ahmed AF, Maarouf A, et al. Randomized controlled trial to compare the safety and efficacy of tamsulosin, solifenacin, and combination of both in treatment of double-J stent-related lower urinary symptoms. Adv Urol 2013;2013:752382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shelbaia A, Elnashar A, Khairy H. MP20-10 Role of anticholinergics (tolterodine) and selective alpha-blocker (tamsulosin) in improving the lower urinary tract symptoms associated with double J ureteral stent placement after ureteroscopy. J Urol 2014;191:e199–200. [Google Scholar]
- [13].Streeper NM, Sivalingam S, Sehgal PD, et al. Does combination therapy with tamsulosin and tolterodine improve ureteral stent discomfort compared with tamsulosin alone? A double-blind, randomized controlled trial. J Urol 2014;191:S161–2. [DOI] [PubMed] [Google Scholar]
- [14].Park J, Yoo C, Han DH, et al. A critical assessment of the effects of tamsulosin and solifenacin as monotherapies and as a combination therapy for the treatment of ureteral stent-related symptoms: a 2 × 2 factorial randomized trial. World J Urol 2015;33:1–8. [DOI] [PubMed] [Google Scholar]
- [15].Sivalingam S, Streeper NM, Sehgal PD, et al. Does combination therapy with tamsulosin and tolterodine improve ureteral stent discomfort compared with tamsulosin alone? A double-blind, randomized, controlled trial. J Urol 2016;195:385–90. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collaboration [updated March 2011]. Available at: www.cochrane-handbook.org Accessed 2016. [Google Scholar]
- [17].Sahebkar A, Serban C, Ursoniu S, et al. Effect of garlic on plasma lipoprotein (a) concentrations: a systematic review and meta-analysis of randomized controlled clinical trials. Nutrition 2016;32:33–40. [DOI] [PubMed] [Google Scholar]
- [18].Dimitropoulos K, Gravas S. Solifenacin/tamsulosin fixed-dose combination therapy to treat lower urinary tract symptoms in patients with benign prostatic hyperplasia. Drug Design Devel Ther 2015;9:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang H, Man LB, Huang GL, et al. Comparative efficacy of tamsulosin versus nifedipine for distal ureteral calculi: a meta-analysis. Drug Des Devel Ther 2016;10:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park SC, Jung SW, Lee JW, et al. The effects of tolterodine extended release and alfuzosin for the treatment of double-j stent-related symptoms. J Endourol 2009;23:1913–7. [DOI] [PubMed] [Google Scholar]
- [21].el Din KE, Koch WF, de Wildt MJ, et al. Reliability of the international prostate symptom score in the assessment of patients with lower urinary tract symptoms and/or benign, prostatic hyperplasia. J Urol 1996;155:1959–64. [DOI] [PubMed] [Google Scholar]
- [22].Roosen A, Wu C, Chowdhury RA, et al. Characteristics of spontaneous activity in the bladder trigone. Eur Urol 2009;56:346–53. [DOI] [PubMed] [Google Scholar]
- [23].Kwon JK, Kang SC, Oh CK, et al. The beneficial effect of alpha-blockers for ureteral stent-related discomfort: systematic review and network meta-analysis for alfuzosin versus tamsulosin versus placebo. BMC Urol 2015;15:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park HK, Paick SH, Kim HG, et al. The impact of ureteral stent type on patient symptoms as determined by the ureteral stent symptom questionnaire: a prospective, randomized, controlled study. J Endourol 2014;29:367–71. [DOI] [PubMed] [Google Scholar]
- [25].Irani J, Siquier J, Pirès C, et al. Symptom characteristics and the development of tolerance with time in patients with indwelling double-pigtail ureteric stents. BJU Int 1999;84:276–9. [DOI] [PubMed] [Google Scholar]
- [26].Beddingfield R, Pedro RN, Hinck B, et al. Alfuzosin to relieve ureteral stent discomfort: a prospective, randomized, placebo controlled study. J Urol 2009;181:499–1499. [DOI] [PubMed] [Google Scholar]
- [27].Jüni P, Egger M. Allocation concealment in clinical trials. JAMA 2002;288:2408–9. [DOI] [PubMed] [Google Scholar]
- [28].Eveline N, Stephan R, Sven T, et al. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta-epidemiologic study. Arthritis Rheumatol 2009;61:1633–41. [DOI] [PubMed] [Google Scholar]
- [29].Lange D, Bidnur S, Hoag N, et al. Ureteral stent-associated complications—where we are and where we are going. Nat Rev Urol 2015;12:17–25. [DOI] [PubMed] [Google Scholar]
- [30].Kicinski M, Springate DA, Kontopantelis E. Publication bias in meta-analyses from the Cochrane Database of Systematic Reviews. Stat Med 2015;34:2781–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


