Despite the high prevalence and severe consequences of calcific aortic valve disease (CAVD), its etiology remains relatively poorly understood. For many years, CAVD was considered a degenerative disease, where calcification was a consequence of normal aging. Although it is now widely accepted that CAVD is an active disease process [1], the erroneous “degenerative” label contributed to delayed progress in characterizing this condition. At present, our understanding of the mechanisms governing CAVD lags far behind our knowledge of atherosclerosis, a disease to which CAVD is frequently compared. Ultimately, our incomplete understanding of CAVD etiology has profound consequences: other than surgical valve replacement, there remains no treatment to stop the onset or progression of CAVD.
Sex as an Important Biological Variable
Consistent with many other cardiovascular diseases, male sex closely follows advanced age as a risk factor for developing CAVD [2]. Over the last 5 years, there has been a surge in the appreciation of sex as a biological variable (SABV), with a report on the importance of sex-specific research reporting issued by the Institute of Medicine and National Academies [3], decisions by several journal publishers to require the reporting of the sex of all tissues and cells in papers, and the 2014 introduction of an NIH policy requiring that SABV be addressed as an aspect of rigor and reproducibility in NIH grants. In the context of CAVD, there is scant information on sex-specific differences in valve biology or pathology. While several studies have demonstrated higher prevalence of CAVD amongst males, investigations that explicitly focus on this issue have concentrated on sex differences in ventricular or vascular dysfunction caused by CAVD [4, 5], rather than the valve itself. Moreover, the majority of in vitro valve investigations use valvular interstitial cell (VIC) cultures that are not separated by sex, or whose sex is unknown. Yet, evidence is emerging that cellular-scale sex differences may also play a role in aortic valve pathology [6].
A significant step toward uncovering sex-specific differences in CAVD was achieved in 2013, when Aggarwal et al. provided initial evidence that male sex impacts not only the probability of developing CAVD, but also the nature of the CAVD. Specifically, for an equivalent degree of aortic stenosis, men had significantly greater valve calcification than women, as measured by multidetector CT (MDCT) imaging of patients [7]. This intriguing discovery sets the stage for the current work by Simard et al. [8], whose research builds upon the earlier finding to uncover further differences in pathology between male and female valves with CAVD.
Aortic Valve Stenosis: More than just Calcification
In their study, Simard et al. [8] merge in vivo quantification of stenosis severity and calcification with ex vivo evaluation of explanted leaflets for evidence of calcification and fibrosis. After controlling for patient comorbidities, study participants were evaluated using echocardiography and MDCT imaging to assess aortic stenosis severity and valve calcification, and to frequency-match patients according to demographics and extent of disease. Following routine valve replacement surgery, explanted valves were weighed, sectioned, and stained for different structural and extracellular matrix components known to be present in diseased valves. Specifically, the authors analyzed valve explants for levels of calcification, fibrosis, collagen content, and both dense and loose connective tissue. The degrees of calcification and fibrosis were semi-quantitatively scored in a blinded analysis of histological sections, and fibrosis was further evaluated by quantifying collagen fiber and connective tissue density in tissue sections. The data were analyzed while controlling for multiple sex-specific factors that could interfere with the interpretation of their results, including differences in body size, aortic valve area, and stenosis severity.
The results from this investigation reveal several striking differences between stenotic valves from men and women. Consistent with the earlier work of Aggarwal et al. [7], MDCT imaging yielded higher scores for aortic valve calcification (AVC) density in men compared to women with a similar degree of stenosis. Moreover, the ratio of aortic valve weight density to AVC density was significantly higher in women than in men, suggesting increased amounts of non-calcified tissue in women. By analyzing explanted valves, the authors were then able to further investigate the identity of this abundant non-calcified tissue, and found significantly increased levels of fibrosis in stenotic valves from women relative to men. In men, the fibrosis score also tightly correlated with the extent of calcification, which was not the case for women. Analyses of collagen fiber and connective tissue density further strengthened the conclusion that female sex is an independent predictor for increased fibrosis in CAVD.
Implications for the Study and Treatment of CAVD
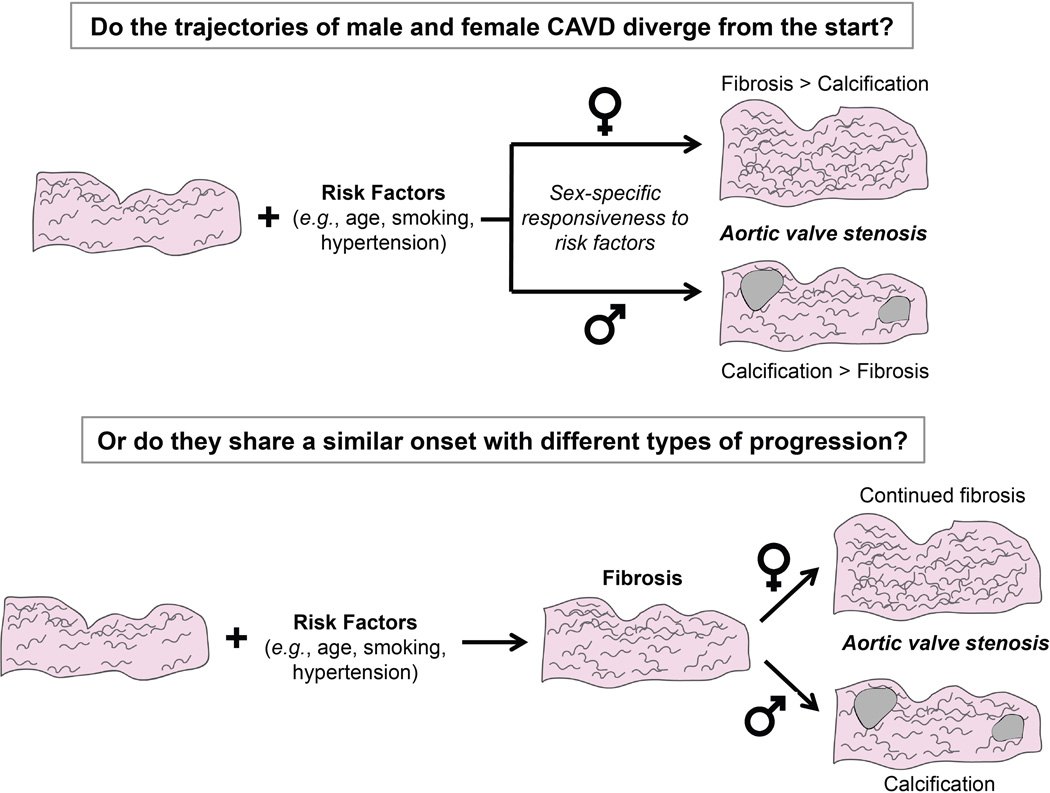
The findings by Simard et al. are remarkable because they suggest that the pathogenesis and extracellular matrix remodeling in aortic valve stenosis may substantially differ with sex. These results raise the question of whether CAVD progresses via a fundamentally different trajectory for men vs. women, or a similar trajectory with different exit points for each sex (Figure 1). Supporting the hypothesis that sex-specific divergence of CAVD etiology may occur early on is a growing body of evidence indicating intrinsic cellular-scale differences between men and women [6] that also yield differences in the cellular response to pathological stimuli. For example, VIC culture in hormone-free osteogenic medium is associated with an increase in calcification in male VICs compared to female VICs [9]. The discovery that men and women exhibit different CAVD pathogenesis has the potential to provoke transformative changes in the approach used to investigate CAVD etiology as well as the clinical treatment of this disease. Additionally, the findings by Simard et al. serve to further differentiate CAVD from atherosclerosis, in which plaque lesions and calcification exhibit similar morphology and pathology across both sexes [10].
Figure 1. Possible routes for sex-specific divergence of CAVD.
Halting the progression of CAVD will require knowledge of whether the disease exhibits sex bias from its onset, or if the initial disease process is shared across sexes, with subsequent differentiation.
The work by Simard et al. also raises the broader issue of what valve characteristics are most appropriate for describing and evaluating CAVD. Research in the field has historically favored the study of calcification over fibrosis, but the female patients included in this study exhibited impaired valve function dominated by fibrosis, rather than calcification. Meanwhile, recent work with a mouse model of hypercholesterolemia and hypertension achieved hemodynamically significant stenosis in the absence of calcification [11]. Thus, beyond identifying sex-related differences in calcification, the study by Simard et al. highlights the need to deepen our understanding of the contribution of fibrosis to CAVD pathogenesis and progression. Furthermore, fibrosis is also associated with age, the primary risk factor for CAVD, where an increase in collagen type I and its cross-linking is observed in aging healthy valves [12]. Understanding the evolution of fibrosis in valves may be a critical step in elucidating the mechanisms that govern both CAVD onset and progression.
A significant limitation of the work presented by Simard et al. is the lack of quantitative data beyond analysis of histological staining to describe fibrosis. Additional analysis approaches are required to characterize the nature of these fibrotic changes and examine potential sex bias in collagen production/degradation dynamics or fiber architecture. Moreover, while Simard et al. make significant progress toward characterizing sex-specific differences in the pathological features of stenotic valves, much remains to be discovered with respect to the mechanisms that govern these differences. As stated by the authors, hormones could play an important role in this process; however, the observation of sex-specific behaviors during in vitro VIC culture suggests additional mechanisms that transcend hormonal effects. Genetic and epigenetic differences are likely involved, and differential production of microRNAs has been proposed as an important regulator of other sex-biased diseases [13]. Deciphering these mechanisms necessitates the decoupling of hormonal and genetic effects, thus emphasizing the need for continued animal and in vitro studies to complement human valve analyses in identifying the origin of these sex-related differences.
The important contributions of the work by Simard et al. underscore the need to continue to conduct prospective and retrospective in vivo and in vitro studies separated by sex to elucidate CAVD etiology. This paper also challenges the assumption that CAVD is defined by end-point calcification, and identifies fibrosis as an important feature of the disease, particularly in women. By expanding our understanding of the nature of CAVD experienced by males and females, this seminal study by Simard et al. provides a foundation for the discovery of genes, proteins, and markers that render male sex the second most important risk factor for this disease. Understanding the manner in which males and females diverge in their development of CAVD will also be critical in generating effective treatments for this disease.
Acknowledgments
The authors acknowledge funding from the National Institutes of Health (R01 HL093281, R21 EB019508) and the American Heart Association (15PRE 22170006).
Footnotes
Disclosures
None.
Cited References
- 1.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105(5):701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wizemann T. Sex-Specific Reporting of Scientific Research: A Workshop Summary. Washington, D.C.: Institute of Medicine of the National Academies, Board on Population Health and Public Health Practice; 2012. [Google Scholar]
- 4.Dobson LE, Fairbairn TA, Musa TA, Uddin A, Mundie CA, Swoboda PP, et al. Sex-related differences in left ventricular remodeling in severe aortic stenosis and reverse remodeling after aortic valve replacement: A cardiovascular magnetic resonance study. Am Heart J. 2016;175:101–111. doi: 10.1016/j.ahj.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Carroll JD, Carroll EP, Feldman T, Ward DM, Lang RM, McGaughey D, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86(4):1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 6.McCoy CM, Nicholas DQ, Masters KS. Sex-related differences in gene expression by porcine aortic valvular interstitial cells. PLoS One. 2012;7(7):e39980. doi: 10.1371/journal.pone.0039980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal SR, Clavel MA, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PA, et al. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6(1):40–47. doi: 10.1161/CIRCIMAGING.112.980052. [DOI] [PubMed] [Google Scholar]
- 8.Simard L, Côté N, Dagenais F, Mathieu P, Couture C, Trahan S, et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ Res. 2017 doi: 10.1161/CIRCRESAHA.116.309306. [DOI] [PubMed] [Google Scholar]
- 9.Masjedi S, Lei Y, Patel J, Ferdous Z. Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells. Heart Vessels. 2016 doi: 10.1007/s00380-016-0909-8. [DOI] [PubMed] [Google Scholar]
- 10.Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239(1):260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Chu Y, Lund DD, Doshi H, Keen HL, Knudtson KL, Funk ND, et al. Fibrotic Aortic Valve Stenosis in Hypercholesterolemic/Hypertensive Mice. Arterioscler Thromb Vasc Biol. 2016;36(3):466–474. doi: 10.1161/ATVBAHA.115.306912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksen HA, Satta J, Risteli J, Veijola M, Vare P, Soini Y. Type I and type III collagen synthesis and composition in the valve matrix in aortic valve stenosis. Atherosclerosis. 2006;189(1):91–98. doi: 10.1016/j.atherosclerosis.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Eghbali M. Influence of sex differences on microRNA gene regulation in disease. Biol Sex Differ. 2014;5(1):3. doi: 10.1186/2042-6410-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]