Abstract
Introduction
Oxytocin is routinely used as prophylaxis against uterine atony. During elective cesarean delivery (CD), an oxytocin bolus is used to initiate adequate uterine tone, followed by an oxytocin infusion to maintain uterine contractility. However, it is unclear whether oxytocin maintenance infusion rate influences total estimated blood loss (EBL).
Methods
We performed a prospective, randomized, double-blind trial in 51 women undergoing elective CD. Women were randomized to receive an oxytocin maintenance infusion of 2.5 u/hr or 15 u/hr. All women received an oxytocin 1 u bolus to initiate adequate uterine tone. The primary outcome was EBL. EBL values between groups were compared using a Mann-Whitney U test; P<0.05 as statistically significant. The median EBL difference with 95% confidence intervals (CI) was also calculated. Secondary outcomes included: adequacy of uterine tone, use of additional uterotonics, and oxytocin related side-effects, including hypotension.
Results
Of 51 women, 24 received a low rate infusion and 27 received a high rate infusion. Median [interquartile range] EBL values in the low rate and high rate groups were: 634 [340 – 886] ml vs. 512 [405 – 740] ml, respectively; P=0.7). The median difference in EBL between groups was 22 ml; 95% CI=−158 – 236 ml. The rate of postpartum hemorrhage did not differ between groups (low rate group: 4/24 (16.7%) vs. high rate group: 4/26 (15.4%)). There were no between-group differences over time (first 20 min after commencing infusion) in the incidence of adequate uterine tone (P=0.72) or hypotension (P=0.32).
Conclusion
Among women undergoing elective CD receiving an oxytocin maintenance infusion, EBL and uterine tone did not differ between women receiving 2.5 u/hr oxytocin to those receiving 15 u/hr oxytocin. Our findings suggest that efficacy can be obtained with a low oxytocin maintenance infusion rate, however dose-finding studies are needed to determine the infusion rate that optimizes drug efficacy whilst minimizing side effects.
Introduction
Oxytocin is the preferred agent for prophylaxis against uterine atony after vaginal or cesarean delivery (CD).1 During the third stage of labor, oxytocin decreases the risk of PPH and the need for therapeutic uterotonics compared to placebo.2 A low dose of oxytocin, delivered either as a bolus or an infusion during elective CD, can initiate adequate uterine tone after placental delivery.3–6 Additionally, important maternal side-effects, such as hypotension, tachycardia, myocardial ischemia, and nausea and vomiting can be minimized with low dose oxytocin regimens.7–10
After initiating adequate uterine tone, an oxytocin infusion is necessary for maintaining adequate uterine contractility. In a randomized controlled trial, women who received an oxytocin bolus followed by oxytocin infusion were found to have a reduced risk of requiring additional uterotonics compared to women who received a bolus with no oxytocin (placebo) infusion.11 However, dose-finding studies have focused on the effective oxytocin infusion rate for initiating adequate uterine tone,5,6 and consensus is lacking on the effective oxytocin infusion rate for maintaining adequate uterine tone during CD. Therefore, investigating the effect of different infusion rates for maintaining adequate uterine tone is of clinical importance for several reasons. Firstly, after initiating uterine contractility, continuation of an identical infusion rate may result in a high incidence of oxytocin related side-effects.6 Using a lower infusion rate may maintain adequate tone whilst minimizing oxytocin related side-effects. Secondly, because of limited evidence, data from national surveys indicates that marked variation exists in prescribed oxytocin regimens during CD.12–14 More studies are needed to provide new evidence for updating clinical guidelines for atony prophylaxis.
To inform clinical practice, we performed a double-blind randomized trial to investigate the effect of a high rate (15 u/hr) vs. low rate (2.5 u/hr) infusion of oxytocin on total estimated blood loss (EBL). Secondary outcomes were: subjective assessments of uterine tone by the obstetrician, use of additional uterotonics, and oxytocin related side-effects.
Methods
We performed a double-blind, randomized trial comparing two oxytocin maintenance infusions during elective CD under neuraxial anesthesia. The study was approved by Stanford University IRB (Protocol 28015), and written informed consent was obtained from the study participants. The study was conducted at Lucile Packard Children’s Hospital, Stanford University. Prior to patient enrolment, the study was registered at clinicaltrials.gov (NCT01932060).
Inclusion criteria were American Society of Anesthesiologists physical class 2, singleton pregnancies, ≥ 37 weeks’ gestational age, elective CD with a Pfannansteil incision, and age between 18 and 40 yrs. All patients received intrathecal anesthesia using a spinal or combined spinal-epidural technique. Exclusion criteria were patients with significant medical or obstetric disease, active labor or ruptured membranes, placenta previa or other placental disorders, multiple gestation, known uterine abnormalities, and allergies to oxytocin.
Prior to surgery, patients were randomized to one of two study groups: a low rate maintenance infusion of oxytocin (oxytocin 10 u in 1 L lactated ringers infused at 250 ml/hr, equivalent to 2.5 u/hr or 0.042 u/min), or a high rate maintenance infusion (oxytocin 60 u in 1 L lactated ringers infused at 250 ml/hr (equivalent to 15 u/hr or 0.25 u/min). Based on data from prior studies,5,6,11,15–17 the selected oxytocin infusion rates were decided a priori by consensus. Opaque envelopes containing group assignments ensured blinding of study investigators, and members of the anesthesia and surgical teams. A computer-generated random code specifying group assignment was contained in each envelope. We used randomly permuted blocks generated by SAS 9.3 (Cary, NC). Oxytocin infusions were prepared by an anesthesiologist not involved in the study.
On the day of surgery, a preoperative hemoglobin (Hb) level was measured. Baseline maternal heart rate (HR) and non-invasive blood pressure (BP) were recorded in the preoperative area on the day of surgery. Mean values for HR and BP were calculated from three measurements. All patients received a 500 ml preload of hetastarch (Hospira; Lake Forest, IL), infused over 30 mins prior to spinal anesthesia. Lactated Ringer’s solution was infused intraoperatively by the primary anesthesia team, with a goal to limit the total crystalloid volume to a maximum of 2 L. Before spinal anesthesia initiation, patients were moved to the sitting position on the operating room table. Spinal anesthesia was induced using hyperbaric bupivacaine 1.6 ml 0.75% bupivacaine, fentanyl 10–15 mcg, and morphine 150–200 mcg. Patients were then moved to the supine position with left lateral displacement. Surgery commenced when a bilateral T5 sensory block to pinprick was confirmed.
After delivery of the infant and clamping of the umbilical cord, all patients received an oxytocin 1 u bolus and the study infusion was commenced. A 1 u bolus dose of oxytocin is above the effective dose in 90% of patients (ED90) for initiating adequate uterine tone,3,4 and is routinely used at our institution for elective CD. The blinded attending obstetrician assessed uterine tone by manual palpation at 2 min intervals. These measurements were made between 2 min and 20 min after starting the study infusion. Uterine tone was assessed by the obstetrician as either adequate or inadequate. If the uterine was deemed ‘inadequate’ at any study time-point, an additional oxytocin 1–2 u ‘rescue’ bolus was administered based on the discretion of the blinded attending anesthesiologist who was providing clinical care. If the patient received a total of ≥ 5 u ‘rescue’ oxytocin and the uterine tone was deemed ‘inadequate’, a second-line uterotonic (methylergonovine maleate, carboprost, or misoprostol) could be administered, at the discretion of the obstetric and anesthesia teams. The study infusion was discontinued when each patient was deemed ready for discharge from the post-anesthetic care unit (PACU).
The primary study outcome was total EBL, measured quantitatively at the end of the intraoperative period by a blinded study investigator (AD, CM) who was not involved in patient care. The study investigator calculated the total EBL using an approach previously described.18 In brief, EBL was calculated by summing the following measurements: total weight of blood on blood soaked surgical laps, measured using electronic scales; estimate of blood contained in the suction canister; and estimate of blood loss around the surgical field and drapes after completion of surgery. We calculated rates of PPH using a traditional definition of EBL>1000 ml.1
Secondary outcomes included: adequacy of uterine tone (yes/no); Hb level at the time of PACU discharge, number of rescue doses of oxytocin, duration of the oxytocin infusion, need for secondary uterotonic agents, use of surgical interventions for blood loss control (including; B-lynch brace suture, intrauterine balloon tamponade, vessel ligation, hysterectomy), use of blood components during surgery or PACU, duration of PACU stay, and hospital length of stay. We also recorded the incidence of clinically relevant oxytocin related side-effects (bradycardia [classified as a HR<40 beats per minute (bpm)], tachycardia [classified as HR≥120 bpm], hypotension [classified as a systolic blood pressure ≤80% of the baseline value], nausea, and vomiting, flushing, headache, chest pain, and dyspnea). The assessment of oxytocin related side-effects were observed by the study investigator at the same time as the uterine tone assessments (between 2 min and 20 min after commencing the infusion).
Statistical Analyses
Data were assessed for normal distribution of variance using the Kolmogorov-Smirnov tests and normality plots. For the baseline characteristics, we assessed whether any clinically relevant between-group differences were apparent. If a clinical imbalance was noted for any baseline characteristic, a multivariable mixed effects model was considered with EBL as the dependent variable and group as a fixed independent variable, with adjustment for relevant baseline characteristics.
To assess the effect of a low vs. high oxytocin infusion rate on EBL (primary outcome), we compared EBL values between groups using the Mann-Whitney U test. We also calculated the median (95% confidence interval (CI)) between-group difference in EBL. For postoperative Hb, duration of infusion, rescue dose of oxytocin, between-group differences were compared using the Mann-Whitney U test and the median between-group difference with 95% confidence intervals (CIs) for each parameter were calculated. The proportion of women in each group with PPH were compared using the Fisher’s exact test, and we compared the 95% CI for the difference in proportions using the 2-sample test for equality in proportions.
To assess the effect of a low vs. high infusion rate on the following secondary outcomes of interest: hypotension, adequacy of uterine tone, ‘rescue’ doses of oxytocin, we used a longitudinal fixed-effects models, based on a generalized estimated equation (GEE) approach. For these models, time and study group were fixed effects. For the longitudinal model assessing hypotension, we adjusted for baseline systolic BP. Data are presented as mean (SD), median [interquartile range], or n (%). We performed statistical analyses using Stata version 12 (College Station, TX). A P<0.05 was considered as statistically significant.
Based on data from prior studies3,6,19 and the combined clinical experience of study investigators, to detect a 250 ml (SD 350 ml) decrease in EBL for women in the high rate compared to the low rate group with a two sided significance of 5% and a power of 90%, 42 patients per group was needed. To account for fallouts, we aimed to recruit 45 patients per group. Analysis was by intention to treat.
Results
Between August 2013 and July 2015, 140 patients were approached and 51 patients completed the study (Figure 1). We halted recruitment in July 2015 for two reasons: slow accrual of patients, and introduction of an important change in clinical practice for women undergoing elective CD, namely phenylephrine infusions for prophylaxis against spinal hypotension. At the time of study termination, we recruited 24 patients in the low rate group and 27 patients into the high rate group. Based on our sample size at the time of study termination (at least 23 patients per group), we had 68% power to detect a 250 ml (SD 350 ml) decrease in EBL between groups. Conversely, 23 patients per group did provide 90% power to detect a 335 ml (SD 350 ml) difference between groups, with a two sided significance of 5%.
Figure 1.
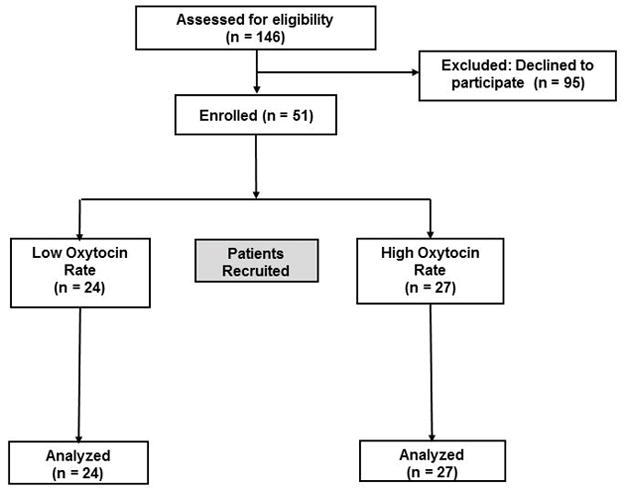
Flow diagram
We observed no clinically relevant between-group differences for any baseline (demographic or obstetric) characteristic (Table 1). Mean preoperative Hb values did not differ between groups: 11.8 (1.3) g/dl vs. 11.9 (1.1) g/dl in the low rate vs. high rate group, respectively. The mean (95% CI) between-group difference in preoperative Hb was −0.1 (−0.8 – 0.6) g/dl.
Table 1.
Baseline and Obstetric Characteristics
| Low Rate Oxytocin Infusion (n=24) | High Rate Oxytocin Infusion (n=27) | |
|---|---|---|
| Maternal age (y) | 32 (5) | 34 (6) |
| Weight (kg) | 81 (12) | 75 (12) |
| Parity | 1 [0–1] | 1 [1–2] |
| Race/Ethnicity: | ||
| Caucasian | 8 (33.3%) | 12 (44.5%) |
| Asian | 4 (16.7%) | 6 (22.2%) |
| Hispanic | 9 (37.5%) | 7 (25.9%) |
| Other | 3 (12.5%) | 2 (7.4%) |
| Gestational age (weeks) | 39 (1) | 39 (1) |
| Number of prior CDs | 1 [0–1] | 1 [1–2] |
| Preoperative Hb (g/dl) | 11.8 (1.3) | 11.9 (1.1) |
Data presented as mean (SD), median [interquartile range], and n (%)
CD=cesarean delivery; Hb=hemoglobin
We observed no statistically significant difference in EBL between groups. Median [IQR] EBL was 634 [340 – 886] ml for the low rate group vs. 512 [405 – 740] ml for the high rate group; P=0.7 (figure 1). The median difference in EBL between groups was 22 ml (95% CI=−158 – 236 ml). EBL was incorrectly measured for one patient in the high rate group, therefore this patient’s data was not included in the between group analysis for EBL. The rate of PPH did not differ between groups: 4/24 (16.7%) in the low rate group vs. 4/26 (15.4%) in the high rate group; P=1.0. The difference in the proportion of women with PPH between groups was 1.3% (95% CI= −49.6% – 52.2%). Median Hb values in PACU were not different between groups: 11.2 [10.3 – 12.2] g/dl in the low rate group vs. 11.1 [9.9 – 11.8] g/dl in the high rate group; P=0.77. The median (95% CI) between-group difference in postoperative Hb was 0.1 (−0.7 – 0.9) g/dl. Median [IQR] duration of study infusion was not different between groups: 90 (90 – 117) min in the low rate group vs. 90 (90 – 100) min in the high rate group; P=0.88. The median difference in duration of infusion between groups was 0 min (95% CI=−2 – 3 min).
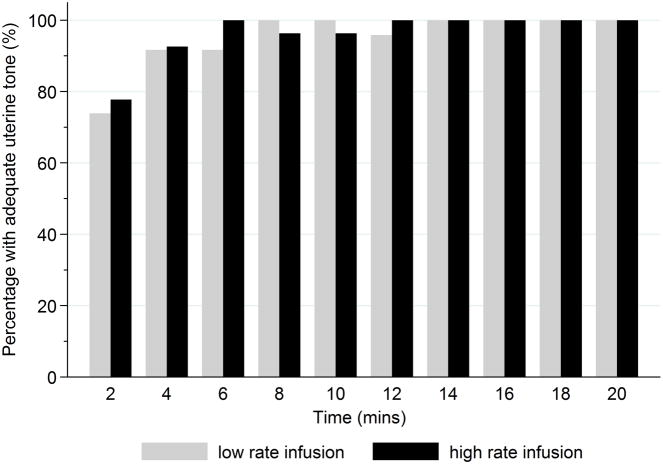
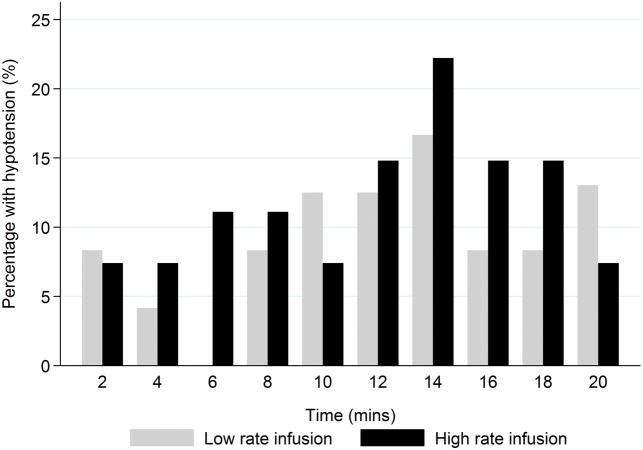
The total dose of ‘rescue’ oxytocin was not different between groups: 1 [1 – 1.5] u in the low rate group vs. 1 [1 – 2] u in the high rate group; P=0.98. The median between-group difference in the ‘rescue’ dose of oxytocin was 0 u. In our GEE model, we observed no significant between-group difference in the rescue dose of oxytocin used over time (P=0.72). One patient in each group required a second-line uterotonic intraoperatively (misoprostol in the low rate group; methylergonovine in the high rate group). No patients required a second-line uterotonic in PACU. We observed no statistically significant between-group differences over time in the proportion of patients who achieved adequate uterine tone (P=0.72) or hypotension (P=0.32) (Figures 2 and 3). The frequencies of other oxytocin related side-effects (nausea, dyspnea, flushing, headache, chest pain, and tachycardia) are presented in Table 2. No patients experienced vomiting or bradycardia in either of the study groups.
Figure 2.
Bar chart depicting the proportion of women with adequate uterine tone at each study time point after commencing study infusion.
Figure 3.
Bar chart depicting the proportion of women with hypotension at each study time point after commencing study infusion.
Table 2.
Oxytocin Side-Effects among Women receiving Low Rate and High Rate Oxytocin Infusions.
| Time after commencing study infusion*
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 min | 4 min | 6 min | 8 min | 10 min | 12 min | 14 min | 16 min | 18 min | 20 min | |
| Nausea | ||||||||||
| Low Rate Infusion | 3 (12.5%) | 3 (12.5%) | 1 (4.2%) | 1 (4.2%) | 1 (4.2%) | 1 (4.2%) | 4 (16.7%) | 3 (12.5%) | 2 (8.3%) | 2 (8.3%) |
| High Rate Infusion | 1 (3.7%) | 1 (3.7%) | 0 | 0 | 1 (3.7%) | 1 (3.7%) | 1 (3.7%) | 0 | 0 | 1 (3.7%) |
| Dyspnea | ||||||||||
| Low Rate Infusion | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.2%) | 0 | 1 (4.2%) | 1 (4.2%) |
| High Rate Infusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flushing | ||||||||||
| Low Rate Infusion | 2 (8.3%) | 3 (12.5%) | 3 (12.5%) | 3 (12.5%) | 3 (12.5%) | 4 (16.7%) | 4 (16.7%) | 2 (8.3%) | 2 (8.3%) | 2 (8.3%) |
| High Rate Infusion | 1 (3.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | ||||||||||
| Low Rate Infusion | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.2%) | 1 (4.2%) | 2 (8.3%) | 2 (8.3%) |
| High Rate Infusion | 0 | 0 | 0 | 0 | 1 (3.7%) | 1 (3.7%) | 0 | 0 | 1 (3.7%) | 1 (3.7%) |
| Chest Pain | ||||||||||
| Low Rate Infusion | 1 (4.2%) | 2 (8.3%) | 2 (8.3%) | 2 (8.3%) | 2 (8.3%) | 1 (4.2%) | 2 (8.3%) | 1 (4.2%) | 1 (4.2%) | 1 (4.2%) |
| High Rate Infusion | 2 (7.4%) | 2 (7.4%) | 2 (7.4%) | 2 (7.4%) | 2 (7.4%) | 1 (7.4%) | 1 (3.7%) | 2 (7.4%) | 2 (7.4%) | 3 (11.1%) |
| Tachycardia | ||||||||||
| Low Rate Infusion | 1 (4.2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High Rate Infusion | 0 | 0 | 0 | 0 | 0 | 1 (3.7%) | 1 (3.7%) | 0 | 0 | 0 |
Data presented as n (%).
Time represents the time-period that has elapsed after commencing the oxytocin maintenance infusion.
One patient in the low rate group incurred bladder wall injury requiring cystectomy. One patient in the low rate group required red blood cell transfusion in PACU for severe postoperative anemia (Hb=6.2 g/dl); she did not incur refractory uterine atony during the perioperative period and her EBL was 918 ml. No patients received any surgical intervention for blood loss control. Two patients in the low rate group had missing postoperative Hb values. Median hospital length of stay was similar in each group: 5 [4 – 5] days in the low rate group vs. 4 [4 – 5] days in the high rate group; the median between group difference in length of stay was 0 days.
Discussion
Our findings suggest that, among healthy women undergoing elective CD, EBL did not differ between women receiving an oxytocin maintenance infusion of 2.5 u/hr compared to those receiving an oxytocin infusion of 15 u/hr. In addition, we observed no between-group differences in the frequencies of PPH. Similarly, no between-group differences were observed over time in the incidence of adequate uterine tone, need for additional uterotonics or hypotension. No between-group differences were observed over time in the dose of rescue oxytocin boluses given after starting the oxytocin infusion.
In recent years, a number of studies have shown that low doses of oxytocin, delivered as a bolus or infusion during elective CD, can successfully initiate uterine contractility while minimizing dose-related side-effects.3–6 After initiating uterine contractility, studies investigating the efficacy of an oxytocin infusion for maintaining adequate uterine tone are limited. Sheehan et al.11 compared an oxytocin 5 u bolus followed by an oxytocin 10 u/hr infusion over 4 hrs vs. a 5 u bolus followed by placebo infusion. They found no between-group differences in the rate of severe PPH or volume of overall blood loss, however women receiving an oxytocin bolus + oxytocin infusion were at reduced odds (OR=0.61) of receiving an additional uterotonic compared to those only receiving an oxytocin bolus followed by a placebo infusion. These findings suggested that there is clinical merit in using a maintenance oxytocin infusion during elective CD. However, in our study, differences in obstetricians’ and anesthesiologists’ preferences for using additional uterotonics or assessment of uterine tone may explain why we observed no between-group differences in additional uterotonic use.
There is a dearth of studies investigating the dose-response of a maintenance oxytocin infusion. This may explain why obstetric societies are inconsistent in their dosing recommendations for oxytocin prophylaxis.1 Data from national surveys suggest that there is wide inter-physician variability in the prescription of oxytocin infusions after oxytocin bolus administration.12–14 In one survey of lead obstetricians and anesthesiologists in the United Kingdom and Ireland, marked variation between countries was reported in the utilization rates of an oxytocin infusion (11–55%), with infusion rates varying from 3.3 u/hr to 20 u/hr.12 Although we observed no between-group differences in our primary or secondary outcomes, the use of a low rate infusion may be advantageous for avoiding cardiovascular instability in high-risk patients, such as women with cardiac disease.
Oxytocin is associated with important cardiovascular and non-cardiovascular side-effects, such as hypotension, tachycardia, ST segment changes, nausea, and vomiting.10,20 The incidence and magnitude of these effects may be dose-related.9 In our study, despite the 6-fold difference between groups in the oxytocin dose infused per hour, we did not observe any significant differences in the incidence of hypotension over time between groups. Oxytocin side-effects were uncommon in both groups and, surprisingly, flushing and nausea occurred more frequently in the low-rate group. Comparisons of side-effect frequencies across studies is challenging because of between-study differences in oxytocin infusion rates.6,16,21
Our study has a number of potential limitations. Our study did not include a placebo group, therefore it is uncertain whether all patients may benefit from an oxytocin maintenance infusion. The results of our a priori and post-hoc power analyses deserve attention for several reasons. Firstly, because of a dearth of studies examining oxytocin regimens for maintaining adequate tone, we based the a priori effect size (SD) estimates on previously published data from studies examining oxytocin regimens for initiating uterine tone.3,6,19 These assumptions may explain why the planned EBL difference (250 ml) fell just outside of the 95% CIs for the observed median EBL difference (−158 ml – 236 ml). However, these limits are the best available evidence of where the true effect of treatment may lie. Secondly, the observed median difference in EBL (22 ml) was substantially smaller than the difference we planned to detect (250 ml). Thirdly, with approximately 23 patients per group, we only had 67% power to detect a 250 ml difference in EBL between groups. To determine the accuracy of our findings, validation studies in larger study populations are needed. Lastly, we focused on low risk women undergoing uncomplicated elective CD. Because women undergoing intrapartum CD require higher oxytocin doses to initiate adequate uterine tone compared to those undergoing prelabor CD,4,6,22 it is possible that those at high-risk for uterine atony need a higher oxytocin infusion rate to maintain uterine contractility.
A number of our secondary clinical outcome measures (adequate uterine tone, bradycardia, tachycardia, hypotension) were recorded as binary data as opposed to continuous data, therefore we are unable to perform area under the curve analyses for these outcomes. Because our study was likely underpowered to examine these secondary outcomes, population-wide studies are needed to investigate whether the infusion rate of oxytocin influences the incidence and severity of these side-effects as well as rates of major hemorrhage-related morbidity, such as transfusion, intensive care admission, and major organ dysfunction.
In conclusion, after initiating adequate uterine tone with an oxytocin bolus, our findings suggest that EBL values do not differ between women receiving an oxytocin infusion of 15 u/hr vs. 2.5 u/hr. Based on these findings, after initiating adequate uterine tone during elective CD, a low rate oxytocin infusion is adequate for maintaining uterine contractility. We observed no additional benefit from using a 6-fold higher infusion rate. Future dose-finding studies are needed to determine the optimal infusion rate of oxytocin for maintaining uterine contractility and minimizing blood loss, while reducing oxytocin-related side effects.
Acknowledgments
Funding: The study was supported by the Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine. A.J.B was supported by an award from the Eunice Kennedy Shriver National Institute of Child Health and Development (K23HD070972). A.J.B. has also received gift funding from Masimo Corp; a portion of this funding was used to cover costs for hemoglobin tests in this study. The other authors report no conflict of interest.
Footnotes
IRB Information: This study was approved by the Stanford University IRB. Contact details: Research Compliance Office, Stanford University, 3000 El Camino Real, Five Palo Alto Square, 4th Floor, Palo Alto, CA 94306, USA.
Clinical Trial Registration: This study was registered at ClinicalTrials.gov. The ClinicalTrials.gov Identifier = NCT01932060. URL: https://clinicaltrials.gov/ct2/show/NCT01932060
References
- 1.Dahlke JD, Mendez-Figueroa H, Maggio L, Hauspurg AK, Sperling JD, Chauhan SP, Rouse DJ. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213:76, e1–e10. doi: 10.1016/j.ajog.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013;10:Cd001808. doi: 10.1002/14651858.CD001808.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective Caesarean delivery. Br J Anaesth. 2010;104:338–43. doi: 10.1093/bja/aeq004. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol. 2004;104:1005–10. doi: 10.1097/01.AOG.0000142709.04450.bd. [DOI] [PubMed] [Google Scholar]
- 5.George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth. 2010;57:578–82. doi: 10.1007/s12630-010-9297-1. [DOI] [PubMed] [Google Scholar]
- 6.Lavoie A, McCarthy RJ, Wong CA. The ED90 of prophylactic oxytocin infusion after delivery of the placenta during cesarean delivery in laboring compared with nonlaboring women: an up-down sequential allocation dose-response study. Anesth Analg. 2015;121:159–64. doi: 10.1213/ANE.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 7.Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology. 2013;119:541–51. doi: 10.1097/ALN.0b013e31829416dd. [DOI] [PubMed] [Google Scholar]
- 8.Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100:683–9. doi: 10.1093/bja/aen071. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG. 2010;117:76–83. doi: 10.1111/j.1471-0528.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 10.Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anaesthesiol. 2011;24:255–61. doi: 10.1097/ACO.0b013e328345331c. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan SR, Montgomery AA, Carey M, McAuliffe FM, Eogan M, Gleeson R, Geary M, Murphy DJ, Group ES. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: double blind, placebo controlled, randomised trial. BMJ. 2011;343:d4661. doi: 10.1136/bmj.d4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan SR, Wedisinghe L, Macleod M, Murphy DJ. Implementation of guidelines on oxytocin use at caesarean section: a survey of practice in Great Britain and Ireland. Eur J Obstet Gynecol Reprod Biol. 2010;148:121–4. doi: 10.1016/j.ejogrb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Marcus HE, Fabian A, Lier H, Dagtekin O, Bottiger BW, Teschendorf P, Petzke F, Valter M, Spohr F. Survey on the use of oxytocin for cesarean section. Minerva Anestesiol. 2010;76:890–5. [PubMed] [Google Scholar]
- 14.West R, West S, Simons R, McGlennan A. Impact of dose-finding studies on administration of oxytocin during caesarean section in the UK. Anaesthesia. 2013;68:1021–5. doi: 10.1111/anae.12381. [DOI] [PubMed] [Google Scholar]
- 15.Munn MB, Owen J, Vincent R, Wakefield M, Chestnut DH, Hauth JC. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2001;98:386–90. doi: 10.1016/s0029-7844(01)01479-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim TS, Bae JS, Park JM, Kang SK. Hemodynamic effects of continuous intravenous injection and bolus plus continuous intravenous injection of oxytocin in cesarean section. Korean J Anesthesiol. 2011;61:482–7. doi: 10.4097/kjae.2011.61.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tita AT, Szychowski JM, Rouse DJ, Bean CM, Chapman V, Nothern A, Figueroa D, Quinn R, Andrews WW, Hauth JC. Higher-dose oxytocin and hemorrhage after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2012;119:293–300. doi: 10.1097/AOG.0b013e318242da74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butwick A, Hilton G, Carvalho B. Non-invasive haemoglobin measurement in patients undergoing elective Caesarean section. Br J Anaesth. 2012;108:271–7. doi: 10.1093/bja/aer373. [DOI] [PubMed] [Google Scholar]
- 19.Kovacheva VP, Soens MA, Tsen LC. A Randomized, Double-blinded Trial of a “Rule of Threes” Algorithm versus Continuous Infusion of Oxytocin during Elective Cesarean Delivery. Anesthesiology. 2015;123:92–100. doi: 10.1097/ALN.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 20.Langesaeter E, Rosseland LA, Stubhaug A. Hemodynamic effects of oxytocin during cesarean delivery. Int J Gynaecol Obstet. 2006;95:46–7. doi: 10.1016/j.ijgo.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 21.King KJ, Douglas MJ, Unger W, Wong A, King RA. Five unit bolus oxytocin at cesarean delivery in women at risk of atony: a randomized, double-blind, controlled trial. Anesth Analg. 2010;111:1460–6. doi: 10.1213/ANE.0b013e3181f8930a. [DOI] [PubMed] [Google Scholar]
- 22.Balki M, Ronayne M, Davies S, Fallah S, Kingdom J, Windrim R, Carvalho JC. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol. 2006;107:45–50. doi: 10.1097/01.AOG.0000191529.52596.c0. [DOI] [PubMed] [Google Scholar]