Abstract
Saposin deficiency is a childhood neurodegenerative lysosomal storage disorder (LSD) that can cause premature death within three months of life. Saposins are activator proteins that promote the function of lysosomal hydrolases that mediate the degradation of sphingolipids. There are four saposin proteins in humans, which are encoded by the prosaposin gene. Mutations causing an absence or impaired function of individual saposins or the whole prosaposin gene lead to distinct LSDs due to the storage of different classes of sphingolipids. The pathological events leading to neuronal dysfunction induced by lysosomal storage of sphingolipids are as yet poorly defined. We have generated and characterised a Drosophila model of saposin deficiency that shows striking similarities to the human diseases. Drosophila saposin-related (dSap-r) mutants show a reduced longevity, progressive neurodegeneration, lysosomal storage, dramatic swelling of neuronal soma, perturbations in sphingolipid catabolism, and sensory physiological deterioration. Our data suggests a genetic interaction with a calcium exchanger (Calx) pointing to a possible calcium homeostasis deficit in dSap-r mutants. Together these findings support the use of dSap-r mutants in advancing our understanding of the cellular pathology implicated in saposin deficiency and related LSDs.
Abbreviations: PSAP, prosaposin; ERG, electroretinograms; LSD, lysosomal storage disease; NPC, Niemann-Pick type C; GFP, green fluorescent protein; RT-PCR, reverse transcription PCR; TEM, transmission electron microscopy; MVB, multivesicular body; MLB, multilamellar body; HSAN1, hereditary sensory and autonomic neuropathy type 1
Keywords: Prosaposin deficiency, Saposin, Lysosomal storage disease, Drosophila, Neurodegeneration, Sphingolipids
Highlights
-
•
Drosophila model of PSD recapitulates neurodegenerative phenotype of human PSD.
-
•
Preferential degeneration of sensory regions correlates with loss of sensory function.
-
•
Sphingosine levels rise with age with an imbalance in sphingosine/ceramide ratios.
-
•
Genetic interaction with the Na +/Ca + exchanger points to a calcium regulation deficit.
1. Introduction
Saposin deficiency is an autosomal recessive lysosomal storage disorder (LSD) that is typically associated with severe, age-dependent neurodegeneration and premature death during early childhood. In humans there are four saposins (saposins A–D), which are encoded by the prosaposin gene (Furst et al., 1988, Nakano et al., 1989, O'Brien et al., 1988). The mature, active saposins are produced by cleavage of the prosaposin precursor during passage through the endosomes to the lysosomes; this function is primarily performed by cathepsin D (Hiraiwa et al., 1997). Once in the acidic lysosome environment, saposins act as activator proteins and promote the function of hydrolases involved in sphingolipid degradation (Azuma et al., 1994, Berent and Radin, 1981, Morimoto et al., 1989, Vogel et al., 1987, Yamada et al., 2004). Mutations in prosaposin therefore cause a primary accumulation of sphingolipid species in the lysosomes. The location and severity of the prosaposin mutation dictates the number of saposins that are affected and hence the degree of sphingolipid accumulation and age of lethality. Mutations abolishing the prosaposin start codon result in an absence of prosaposin and therefore all 4 saposins (OMIM #611721); this causes the most severe pathology and individuals present with severe neurodegeneration at birth and die within 4 months (Elleder et al., 1984, Harzer et al., 1989, Hulkova et al., 2001). Of the single saposin disorders, saposin A deficiency is the most severe and results in death at 8 months old ((Spiegel et al., 2005); OMIM #611722), whereas the mildest of the saposin C mutations cause non-neuronopathic disorders with relatively mild symptoms until the fourth decade of life ((Tylki-Szymanska et al., 2007); OMIM #610539). No single saposin D deficiencies have been reported in humans.
Because each saposin generally promotes the function of a specific sphingolipid hydrolase, the single saposin deficiencies resemble the pathology caused by mutations in their cognate hydrolase (e.g. (Christomanou et al., 1986); reviewed in (O'Brien and Kishimoto, 1991)); total prosaposin deficiency encapsulates many aspects of the single saposin deficiencies but to a more severe degree (Elleder et al., 1984, Harzer et al., 1989, Hulkova et al., 2001).
The sphingolipdoses form the largest group of LSDs, yet to date only one sphingolipidosis, Niemann-Pick Type C (NPC), has been modelled in Drosophila (Fluegel et al., 2006, Huang et al., 2005, Huang et al., 2007, Phillips et al., 2008). To broaden our understanding of the sphingolipidoses, and to help identify pathological events subsequent to sphingolipid storage, we generated a Drosophila model of saposin deficiency. The Drosophila Saposin-related (dSap-r) locus encodes a protein predicted to contain eight saposin-like domains, each containing the classic six-cysteine arrangement found in all mammalian saposins. Characterisation of dSap-r mutants revealed pathology similar to that of the human disorders, including reduced longevity, progressive neurodegeneration, aberrant sphingolipid levels, and physiological deterioration; all hallmark signs of lysosomal storage. Our analysis reveals a genetic interaction with the Na+/Ca+ exchanger, CalX, and suggests a deficit in calcium regulation in the Drosophila model of saposin deficiency.
2. Materials and methods
2.1. Identification of Drosophila Sap-r
A blastp search (NCBI; www.ncbi.nlm.nih.gov) was performed to identify the Drosophila melanogaster prosaposin (PSAP) homologue. The entire Homo sapiens PSAP protein sequence (CAG33027) was used to search the D. melanogaster protein database. Standard blastp assumptions were applied. A reciprocal search was performed, using the D. melanogaster d-Sap-rPA sequence to blast the H. sapiens protein database, to ensure the correct homologue was identified.
2.2. Identification of Drosophila Sap-r monosaposins
To identify the putative monosaposins encoded by the dSap-r gene, each human monosaposin sequence (RCSB Protein Data Bank entries 2DOB, 1N69, 2GTG, 2RB3) was aligned against the full-length dSap-rPA sequence using the bl2seq tool at NCBI. The H. sapiens and D. melanogaster monosaposins were aligned using default settings in ClustalX.
2.2.1. Drosophila stocks
All experimental crosses were grown on maize-meal fly food at 25 °C. Newly enclosed flies were transferred to standard yeast-sucrose-agar fly food. The wild type (+/+) control for all experiments was w1118 crossed to Canton-S. The dSap-rPBac and Df(3R)tll-e (subsequently referred to as a ‘deficiency’ or Df, a large deletion of the genomic region encompassing the dSap-r locus) stocks were from the Bloomington stock centre and the dSap-rNP7456 stock was from the Kyoto stock centre. The dSap-rC27 deletion was generated for this study by mobilising the P-element from the dSap-rNP7456 parent line. The UAS-dSap-r transgenic stock was generated for this study as follows. Briefly, pUAST-dSap-r was generated by excision of dSap-r cDNA from the pOT2 vector (clone GH08312, BDGP Gold collection), using XhoI and EcoRI followed by ligation into the pUAST vector. pUAST-dSap-r was microinjected into w1118 embryos with helper plasmid ∆ 2–3. UAS-mCD8GFP and 1407-GAL4 stocks were kindly provided by Andreas Prokop (University of Manchester, UK).
2.2.2. Longevity
Newly eclosed flies were collected in separate-sex vials of approximately 10 flies/vial and aged at 29 °C. Flies were transferred to fresh food every 2–3 days and the number of surviving flies recorded. Longevity was plotted as the percentage of Day 0 flies alive each subsequent day.
2.2.3. Immunohistochemistry
For the dSap-r expression pattern, third instar larvae were dissected and fixed in 3.7% formaldehyde/PBS for 10 min, followed by 3 × 5 min washes in 0.1% PBST (PBS with 0.1% Triton X-100). Larvae were labelled overnight at 4 °C with mouse anti-repo-8D12 or mouse anti-elav-9F8A9 diluted in PBST (1:50; Developmental Studies Hybridoma Bank, University of Iowa). Washes were performed as above, followed by incubation for 2 h at RT in Cy3-conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch). Larvae were washed (as above) and left in 70% glycerol/PBS for 1–2 h before mounting in Vectashield (Vector Laboratories). To label lysosomes, aged adult brains were dissected in 4% paraformaldehyde/PBS and transferred to fresh fixative for 20 min. Brains were washed 3 × 15–20 min in 0.3% PBST followed by incubation overnight at RT with rabbit anti-Arl-8 (1:500; kindly provided by Debbie Smith, University of York, U·K) and mouse anti-elav (1:50). Brains were washed as above, followed by incubation for 3 h at RT in FITC-conjugated goat anti-rabbit IgG and Cy3-conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch). Brains were washed and mounted in Vectashield. All images were acquired using a Zeiss LSM 510 meta Axiovert 200M laser scanning confocal microscope.
2.2.4. Fly head processing for light microscopy
Aged flies were briefly dipped in 30% ethanol before being submerged in fixative (4% paraformaldehyde, 1% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4) and pinned through the abdomen. The proboscis and accessible air sacs were rapidly removed from the heads. Heads were transferred to glass vials containing fresh fixative and were vacuum treated to remove trapped/adherent air. Vacuum-treated heads were incubated in fresh fixative overnight at 4 °C. All incubations were performed on a rotating wheel, unless otherwise stated. Heads were washed 3 × 10 min in 0.1 M sodium phosphate buffer and post-fixed in 1% OsO4 for 1 h. Following washes in 0.1 M sodium phosphate buffer (3 × 10 min) and dH2O (3 × 10 min), heads were dehydrated in an acetone series (30%, 50%, 70%, 90%, 3 × 100%; 20 min each). Heads were incubated in increasing concentrations of Spurr's resin:acetone (25%, 50%, 75%, 95%, 2 × 100% [at 37 °C]; 45 min each) followed by incubation in 100% resin overnight at 4 °C without rotation. Heads were embedded in Spurr's resin (Spurr, 1969) for 24 h at 70 °C. Three semi-thin serial sections (1.0 μm; Leica Ultracut UCT) were taken every 10–20 μm until the desired depth was reached. Sections were dried onto glass slides, stained with 0.6% toluidine blue in 0.3% sodium carbonate on a hot plate (80 °C) and rinsed with dH2O. Sections were imaged using a Zeiss Axiovert 200 microscope equipped with a Zeiss AxioCam HRm camera.
2.2.5. Quantification of vacuole number
Vacuoles, transparent clearings in the neuronal tissue, were quantified manually under light microscopy for the antennal lobes and the visual system (eye, lamina, medulla, lobula and lobula plate) from 3 serial sections per fly head. The sections were matched for depth through the head. Vacuoles were counted from both sides of the head. ANOVA statistical tests were performed using SPSS software (IBM Corp., USA).
2.2.6. Transmission electron microscopy
After reaching the desired depth by semi-thin sectioning, the same embedded heads used for light microscopy were sectioned for transmission electron microscopy. Ultrathin sections (60–70 nm) were collected on 200 and 400 mesh coated grids, treated with uranyl acetate in 50% ethanol for 10 min and submerged in dH2O to wash. Sections were stained with lead citrate for 10 min in the presence of sodium hydroxide pellets, followed by washing in dH2O. Images were captured using analysis software on a TECNAI G2 (Version 2.18) transmission electron microscope (120 kV).
2.2.7. Neuronal soma area quantification
All TEM micrographs were imaged from the cortex surrounding the antennal lobes (see lower panel in Fig. 3A). Neuronal soma area was quantified using ImageJ software. The soma and nuclear boundary of each neuron were demarcated and the area calculated by first inputting the number of pixels per micron then using the ImageJ area measurement tool. Neuronal soma area were normalised to nuclear area. Pseudocolour images were produced using Adobe Illustrator CS4. Student's t-tests were performed in Microsoft Excel to determine statistical significance.
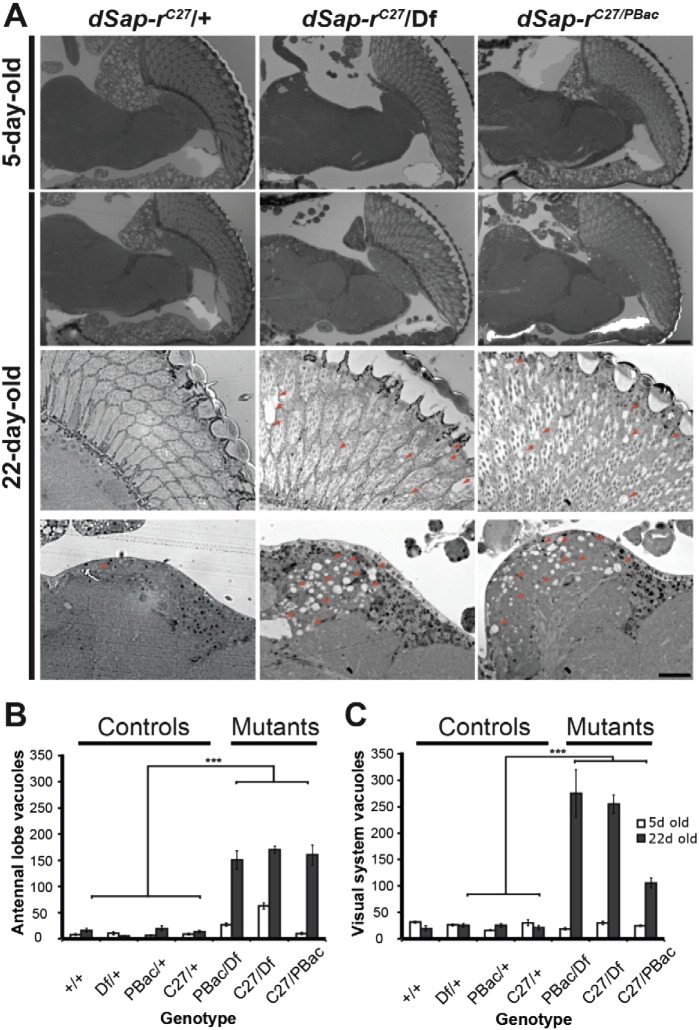
Fig. 3.
Progressive neurodegeneration in sensory regions of dSap-r mutants. A. Representative images of 1 μm sections through 5-day-old and 22-day-old control (dSap-rC27/+) and dSap-r mutant (dSap-rC27/Df and dSap-rC27/PBac) heads are shown at 20 × magnification (top 2 rows) and 63 × magnification (bottom 2 rows, eye (upper row) and antennal lobe (lower row)). Scale bars: 100 μm. B & C. Quantification of vacuole number was performed on 3 serial sections per fly (n ≥ 3 flies). Vacuoles were counted for the visual (B; eye, lamina, medulla and lobula complex) and olfactory (C; antennal lobes) systems at both sides of the brain. Quantification is shown for all controls and dSap-r mutants tested. Genotypes tested: +/+ (wild type); Df/+, PBac/+ and C27/+ (dSap-r heterozygotes); PBac/Df, C27/Df and C27/PBac (dSap-r mutants). Error bars: ± sem. ***p ≤ 0.001.
2.2.8. Lipid extraction and lipidomics analyses
Female adults of the required genotypes were collected on emergence and aged for 5 days. Three brains/biological replicate were dissected in PBS, flash-frozen in 20% methanol using liquid nitrogen, and stored at − 80 °C until lipid extraction was performed. Brain samples were homogenized and extracted according to the methyl-tert-butyl ether extraction method (Matyash et al., 2008). All lipid standards were added to the homogenates prior to extraction (Supplemental Table 1). After phase separation, the organic phase was used for lipidomics and the aqueous phase was processed for determining total protein content using the bicinchoninic acid (BCA) assay (BCA1 kit, Sigma).
For Mass Spectrometry measurements, the samples were dissolved in 100 μl methanol containing 0.1% ammonium acetate and subsequently analyzed using a flow-injection system at a flow rate of 1 μl/min and 5 μl sample injection. Negative and positive ion mode spectra were acquired with a LTQ-Orbitrap XL (Thermo Fisher Scientific, Germany) equipped with an Agilent 1200 micro-LC system (Agilent Technologies, USA) and a Nanomate Triversa utilizing 5 μm ID ESI-chips (Advion, Biosciences, USA).
In the negative mode Phosphatidylinositol (PI), Phosphatidylethanolamine (PE), Polyethylene oxide (PE-O), Lysophosphatidylethanolamine (LPE), Phosphatidylcholine (PC), Ceramide phosphorylethanolamine (CerPE), Phosphatidylserine (PS), Phosphatidylglycerol (PG) could be identified according to their accurate mass (Schwudke et al., 2011). For PS, the specific neutral loss of 87 Da was monitored in the linear ion trap and used for quantification. In the positive mode Sph 14:1, Ceramides and HexCeramides were monitored with MS3 in the linear ion trap using the long chain base related fragment ions. All MS3 for quantifying sphingolipids were analyzed using Xcalibur software 2.07 (Thermo Fisher Scientific, Germany) while all other analyses were performed using LipidXplorer 1.2.4 (Herzog et al., 2011). Absolute levels of individual lipid species (pmol/μg protein) were summed to arrive at lipid class abundances. A minimum of 4 replicates were used for the analyses. Prism 6 software (Prism Software Corp., USA) was used for graph representation and for determining significant differences applying ANOVA coupled with post-hoc Bonferroni tests.
2.2.9. RT-PCR
RNA was extracted from third instar larvae using a QIAGEN RNeasy kit, according to manufacturer's instructions. RNA was treated with DNase prior to cDNA generation. The following primers were used for RT-PCR:
5′TCCTACCAGCTTCAAGATGAC3′ (rp49 Forward), 5′GTGTATTCCGACCACGTTACA3′ (rp49 Reverse), 5′GCAACTGCAACCTGCTTTCC3′ (dSap-r Forward) and 5′GCATCGTTTCCACCATGTCA3′ (dSap-r Reverse).
All PCR reactions using rp49 and dSap-r primers were performed with an elongation time of 1 min, an annealing temperature of 50 °C and 55 °C, and a cycle number of 25 and 35, respectively. dSap-r primers anneal downstream of the dSap-rC27 deletion, but upstream of the dSap-rPBac insertion.
2.2.10. Western blotting
Soluble protein was extracted from aged flies using 10 μl lysis buffer per fly (150 mM NaCl, 20 mM Tris-HCl, pH 8.0, 2 mM EDTA, 0.5% (v/v) NP-40, 1 complete mini protease inhibitor cocktail (Roche)). A standard western blotting procedure was followed including blocking in 5% milk/TBST (Tris buffered saline supplemented with 0.1% (v/v) Tween-20) followed by incubation in primary then secondary antibody diluted in 5% milk/TBST. Washes were performed using TBST. The following antibodies were used: rabbit anti-Arl-8 (1:2000; kindly provided by Debbie Smith, University of York, UK), mouse anti-β-tubulin E7 (1:100; Developmental Studies Hybdridoma Bank, University of Iowa, USA), mouse anti-cathepsin-L (1:250; R&D Systems), hoseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:6000; Sigma) and HRP-conjugated goat anti-mouse (1:10,000; Sigma). Bands were visualised using ECL reagent (GE Healthcare, UK) and developed using a Xograph machine. Densitometry was performed using ImageJ. Band intensities were first normalised to the tubulin control and then to the 5d old wild type control.
2.2.11. Behavioural analyses
The climbing ability of female flies was assessed by tapping cohorts of 5 flies to the bottom of a 100 ml glass measuring cylinder and video recording the climbing response over 45 s. The data were analyzed by quantifying the climbing speed of each fly over 10% intervals of the measuring cylinder. The maximum speed for each fly was used to calculate the average speed per genotype. The same flies tested at 5-days old were re-tested at 22-days old, when possible. ANOVA statistical tests were performed using SPSS software (IBM Corp., USA).
2.2.12. Electroretinograms
ERGs were performed as described in (Hindle et al., 2013). Briefly, aged female flies were left to climb a trimmed 200 μl pipette tip and were blown to the end leaving the fly head protruding. The fly was fixed into position with nail varnish. Glass electrodes were pulled and filled with Drosophila Ringer solution (0.13 M NaCl, 4.7 mM KCl, 1.9 mM CaCl2) (34). A recording electrode was placed against one eye and a reference (earth) electrode placed against the proboscis using micromanipulators. The flies were dark-adapted for 5 min (Fig. 6B - C) or 2 min (Fig. 6D–E). ERGs were recorded in response to 5 × 750 ms blue light pulses with 10 s intervals. Light pulses were provided by a blue LED lamp (Kingbright, Taiwan) controlled by DASYLab software (Measurement Computing Corp., USA). ERGs were analyzed using DASYView software (customised software, C. Elliott freely available download, http://biolpc22.york.ac.uk/dasyview).
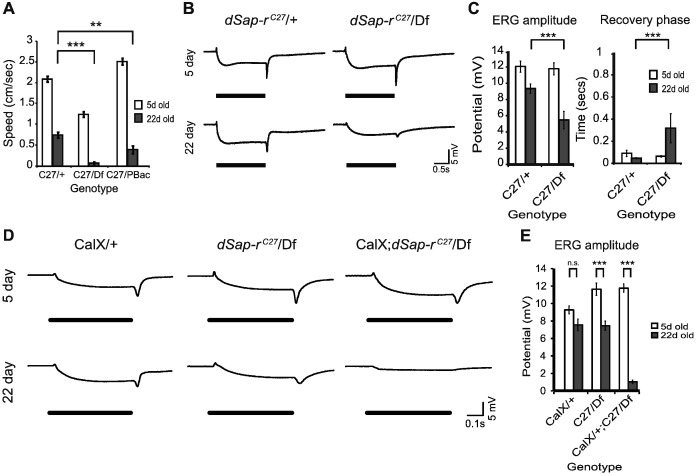
Fig. 6.
Calcium homeostasis defects are associated with progressive deterioration of visual function in dSap-r mutants. A. 5-day-old (white bars) and 22-day-old flies (grey bars) were subjected to climbing assays (n > 20 flies). The climbing speed of dSap-rC27 heterozygotes (C27/+), dSap-rC27/Df (C27/Df) and dSap-rC27/PBac (C27/PBac) mutants are shown. Error bars: ± sem. **p < 0.005 and ***p ≤ 0.001. B. Quantification of electroretinogram (ERG) amplitude and recovery rate after a blue light pulse. The recovery rate is the time taken for the potential to reach half way between the off-transient and base-line potentials after termination of the light pulse. C. Representative ERG traces for 5-day-old and 22-day-old control (dSap-rC27/+) and dSap-rC27/Df mutant females following a blue light pulse. D. Quantification of ERG amplitude after a blue light pulse in 5-day-old and 22-day-old wild type overexpressing the calcium exchanger CalX in the eye (CalX/+), dSap-rC27/Df mutants (C27/Df) and dSap-rC27/Df mutants overexpressing CalX in the eye (CalX/+;C27/Df). E. Representative ERG traces for 5-day-old and 22-day-old wild type overexpressing CalX (CalX/+), dSap-rC27/Df mutants and dSap-rC27/Df mutants expressing CalX (CalX; dSap-rC27/Df). N ≥ 7 flies per condition. *** p ≤ 0.001.
3. Supplemental methods
3.1. Epifluorescence
Organs were dissected from adult flies in PBS and, in some cases, stained with DAPI. Organs were imaged using a Zeiss stereomicroscope equipped with an Axiocam MRc5 camera, a Neolumar S 1.5 × FWD 30 mm lens and a HBO 100 mercury lamp.
4. Results
4.1. dSap-r has homologous sequence structure and similar expression pattern to mammalian prosaposin
The Drosophila prosaposin (PSAP) homologue (Saposin-related; dSap-r) was identified by a BLAST screen using the complete human PSAP protein sequence (blast E value 4e − 36). The dSap-r locus is located at band 100A7 of the right arm of the third chromosome. There are two predicted transcripts for dSap-r (dSap-r RA and dSap-r RB); dSap-r RB appears to be an in-frame truncation of dSap-r RA (Fig. 1A).
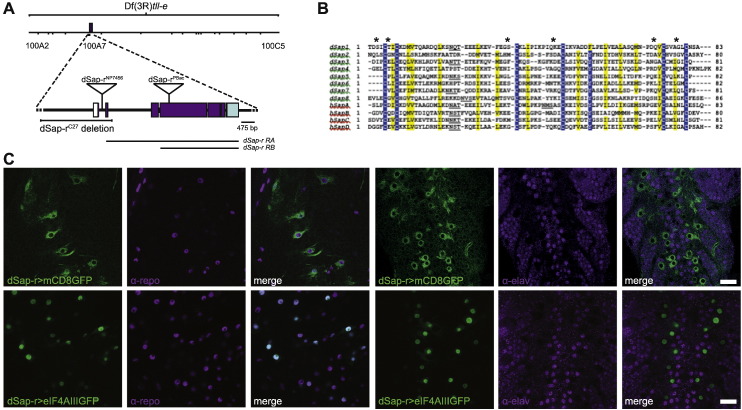
Fig. 1.
Drosophila Sap-r is expressed in glia. A. The Drosophila prosaposin homologue Saposin-related (dSap-r) consists of seven exons and is present on the right arm of the third chromosome (3R) at position 100A7. The dSap-r gene contains two potential transcript start sites (ATG), which result in the production of a transcript consisting of the entire coding sequence (dSap-rRA, 3451 bp) and an in-frame, truncated version (dSap-rRB, 3349 bp) (black lines). The dSap-rC27 mutant allele was generated by P-element mobilisation from the P{GawB}NP7456 line and is a 2.5 kb deletion. The dSap-rPBac mutant allele consists of a 5.971 bp piggyBac transposon insertion into the largest dSap-r exon. The deletion in the deficiency line Df(3R)tll-e spans cytogenetic bands 100A2–100C5, which includes the dSap-r gene. B. A Clustal X alignment of the 8 predicted Drosophila melanogaster saposins (dSaps) and the 4 Homo sapiens saposins (hSaps). Asterisks, conserved cysteine residues; underlining, glycosylation sites in each hSap and possible homologous sites in the dSaps. Blue highlights identical residues; yellow highlights similar residues (80% threshold setting). C. Confocal images of third instar larvae brains expressing mCD8eGFP or eIF4AIIIGFP (n > 5) under the control of dSap-rNP7456 GAL4 and stained with α-repo (glial nuclear marker) or α-elav (pan neuronal marker). Scale bars: 20 μm.
A bl2seq alignment of each human monosaposin (Sap A–D) with the dSap-r protein revealed eight homologous Drosophila saposins (dSaps 1–8). Each dSap contained the six conserved cysteine residues critical for saposin function (Fig. 1B). Five of the dSaps also contained a potential glycosylation signal between the second and third cysteines, previously shown in mammals to be necessary for correct saposin folding and function (Hiraiwa et al., 1993b).
Saposin proteins are expressed in the mammalian nervous system and their loss usually causes severe neurodegeneration (Sun et al., 1994, Van Den Berghe et al., 2004, Yoneshige et al., 2009). We therefore investigated the expression pattern of dSap-r in the nervous system. The dSap-rNP7456 GAL4 enhancer-trap insertion was used to drive a membrane localised GFP reporter (mCD8GFP) in a dSap-r-specific expression pattern. Third instar larvae were dissected and stained with either a glial or neuronal antibody (α-repo or α-elav, respectively). The membrane GFP reporter localised around repo-positive nuclei, suggesting that dSap-r is expressed in glial cells (Fig. 1C). The GFP reporter was also shown to faintly surround elav-positive neuronal nuclei, however this is likely to represent expression in glial cells that surround the neuronal cell bodies. To confirm whether dSap-r is expressed in neurons, a nuclear GFP reporter (eIF4AIII:GFP) was driven by the dSap-rNP7456 element. No colocalisation was found between the nuclear GFP reporter and neuronal nuclei, suggesting that neurons have no or very little dSap-r expression (Fig. 1C). The nuclear GFP colocalised with the glial nuclear marker, further confirming dSap-r expression in glia.
In mammalian visceral organs, prosaposin is expressed ubiquitously at low levels; however moderate to high expression levels are found in the jejunum and tubular epithelial cells in the kidney cortex, epithelial cells of the oesophagus, pancreatic duct and bile duct, and the hepatocytes of the liver (Sun et al., 1994). PSAP has also been shown to promote spermiogenesis and fertility (Amann et al., 1999, Hammerstedt, 1997). Using the mCD8GFP reporter in conjunction with the dSap-rNP7456 enhancer-trap element, dSap-r was shown to be highly expressed in the reproductive system, the digestive system, Malpighian tubules (Drosophila kidney), and the fat bodies (Drosophila liver and adipose tissue) of the adult fly (Fig. S1).
The similar expression pattern of dSap-r and mammalian saposins in cells of the nervous, reproductive, digestive, and renal systems, and the liver is suggestive of conserved functions, and therefore supports the use of Drosophila to model these disorders.
4.2. dSap-r mutation causes a reduced longevity
Patients with saposin deficiency die prematurely, usually within the first decade of life. To test whether dSap-r mutants also die prematurely, we first generated deletions of the dSap-r locus via an imprecise P-element mobilisation strategy. The dSap-rC27 allele is a deletion of the first two exons of the dSap-r locus (Fig. 1A). We also identified a PiggBac transposon insertion, dSap-rPBac, in the 4th exon of the dSap-r locus (Fig. 1A). To assess the dSap-r transcript levels in the dSap-r mutants, RT-PCR was performed (Fig. 1A and 2C). The dSap-r transcript was almost undetectable in dSap-rC27/Df mutants and is likely to reflect dSap-rRB levels, as the dSap-rRA start site is deleted in this mutant. In contrast, dSap-r transcript levels in the dSap-rPBac mutant were indistinguishable from wild type; however, the dSap-r primers were designed to anneal upstream of the dSap-rPBac insertion. Production of saposins from the dSap-r locus prior to the PBac transposon insertion is therefore possible.
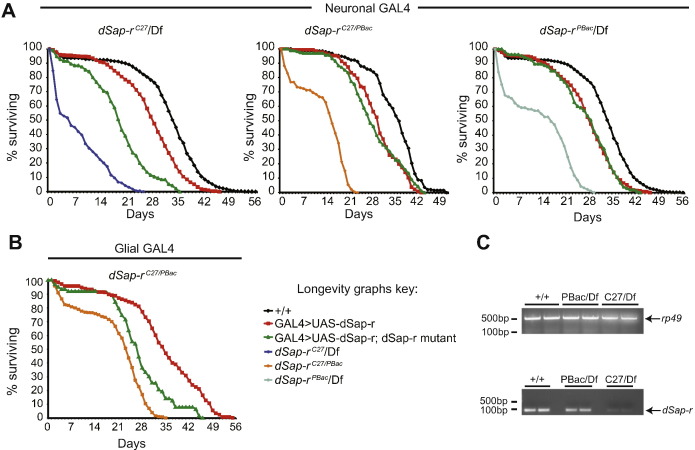
Fig. 2.
Reduced longevity in dSap-r mutants. A & B. Longevity of flies with different dSap-r mutant allelic combinations was assessed at 29 °C. Rescue of dSap-r mutant longevity was performed by expressing dSap-r cDNA in the neurons (A) or glia (B) of dSap-r mutants using the 1407- and repo-GAL4 drivers. Neuronal or glial expression of dSap-r in a wild type background was performed as a transgene control. Longevity was plotted as the percentage of Day 0 flies surviving for each genotype. n > 60 flies for each genotype. C. cDNA from wild type (+/+), dSap-rPBac/Df (PBac/Df) mutant and dSap-rC27/Df (C27/Df) mutant third instar larvae were amplified using rp49 control and dSap-r primers.
Next we tested the effect of these mutant dSap-r alleles using, longevity assays. Each of the three dSap-r mutant allelic combinations caused a reduction in longevity compared to wild type flies (Fig. 2A). Median survival for wild type flies was approximately 35 days, whereas 50% of dSap-r mutants survived only 6 days (dSap-rC27/Df), 15 days (dSap-rC27/PBac) or 18 days (dSap-rPBac/Df). This suggests that the dSap-rC27 mutant allele is more severe than the dSap-rPBac mutant allele. Taken together with the RT-PCR results, this suggests that the dSap-rC27 mutant allele is likely a strong loss-of-function mutation, whereas the dSap-rPBac mutant allele may produce dSap-r protein with reduced or aberrant function.
To confirm that the dSap-r mutations were responsible for the reduced longevity, flies carrying a UAS-dSap-r transgene were generated to allow GAL4-driven rescue of dSap-r mutant longevity. Ubiquitous expression of dSap-r using either Act5c-GAL4 or tubulin-GAL4 resulted in lethality prior to third instar; we therefore used dSap-r expression driven by the neuronal 1407-GAL4 (Fig. 2A) or the glial repo-GAL4 (Fig. 2B) for rescue experiments. Expression of dSap-r in neurons of all dSap-r mutant combinations resulted in a substantial rescue of longevity and in most cases longevity was equivalent to the transgene control. Glial dSap-r expression was only driven in the dSap-rC27/PBac mutants, due to greater genetic ease of repo-GAL4 recombination using the dSap-rPBac mutant allele. Glial dSap-r expression provided a substantial rescue of dSap-rC27/PBac mutant longevity, however, not to the same degree as the neuronal 1407-GAL4 expression. These results confirm that the reduced longevity in dSap-r mutants was due to dSap-r mutations.
4.3. Age-dependent neurodegeneration in dSap-r mutants
The Saposin-deficiencies are primarily classed as progressive neurodegenerative disorders. Therefore, we next assessed whether our dSap-r model showed age-dependent neurodegeneration. In Drosophila, vacuolisation of the brain, the appearance of clearances within the neural tissue is a hallmark of neurodegeneration (Dermaut et al., 2005, Phillips et al., 2008), which can be quantified from 1 μm tissue sections at the light microscopy level. Horizontal sections were taken from 5-day and 22-day-old fly heads and stained with toluidine blue. Vacuolisation was specifically observed in regions of sensory function, mainly the antennal lobes, the eye, and optic lobes (Fig. 3A). Vacuole number was quantified for these sensory regions (Fig. 3B & C) where we observed that vacuole number in most 5-day-old dSap-r mutants was not significantly different from controls, with the exception of limited vacuolisation observed in the antennal lobes of the dSap-rC27/Df mutants. As the flies aged, the number of vacuoles increased in all dSap-r mutants, indicative of progressive neurodegeneration. In the visual system, vacuole number increased by 4–18 fold in the dSap-r mutants compared to less than a 1.5 fold increase in the controls. In the olfactory system (antennal lobes), the controls showed a modest 1.5–3-fold increase in vacuole number. In contrast, the dSap-rPBac/Df and dSap-rC27/PBac mutants showed a massive 6-fold and 16-fold increase in vacuole number, respectively. Although the dSap-rC27/Df mutants showed the greatest number of vacuoles at 22-days old, the increase was only 3-fold due to the increased severity of vacuolisation at 5-days old.
4.4. dSap-r mutants have age-dependent lysosomal storage defects
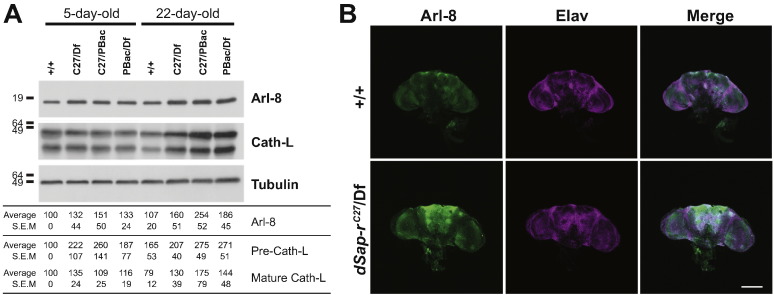
LSDs are characterised by lysosomal dysfunction leading to the storage of undegraded material in the lysosomes (Jardim et al., 2010). Therefore, to assess the degree of lysosomal dysfunction and storage in dSap-r mutants, western blotting was performed using antibodies for two lysosomal proteins: Arl-8 and cathepsin-L.
Arl-8 is an Arf-like GTPase that localises to the lysosomes (Hofmann and Munro, 2006). Cathepsin-L is a lysosomal enzyme that is synthesised as an inactive precursor and cleaved in the lysosome to form its mature, active form (Erickson, 1989). In 5-day-old flies, Arl-8 levels were similar between wild type and dSap-r mutants. However, in 22-day-old dSap-r mutant flies, Arl-8 levels were increased compared to wild type and the 5-day-old samples (Fig. 4A). Similarly, an increase in the mature form of cathepsin-L was found in all 22d old dSap-r mutants. This age-dependent increase in Arl-8 and mature cathepsin-L storage is show by densitometry quantification, however these data did not reach significance due to a variability in the degree of storage in the dSap-r mutants. These data suggest a trend of age-dependent dysfunction and accumulation of lysosomes and/or lysosomal enlargement/swelling in dSap-r mutants, which is consistent with the human disease.
Fig. 4.
Progressive lysosomal storage in dSap-r mutants. A. Western blots showing the abundance of the lysosomal proteins Arl-8 and cathepsin-L in 5-day-old and 22-day-old wild type (+/+), dSap-rC27/Df (C27/Df), dSap-rC27/PBac (C27/PBac) and dSap-rPBac/Df (PBac/Df) mutants (n = 2). Tubulin abundance is shown as a loading control. Average band intensities and S.E.M are shown below. B. Confocal micrographs of 22-day-old wild type (+/+) and dSap-rC27/Df mutant brains showing Arl-8 localisation and abundance (n > 8). Elav staining shows the similar orientation of both wild type and dSap-rC27/Df mutant brains. Scale bar: 100 μm.
In addition to western blotting, the Arl-8 antibody was used for immunohistochemistry on wild type and dSap-rC27/Df mutant adult brains. An overall increase in fluorescence was observed in the aged dSap-r mutant brains, particularly in central brain regions and the antennal lobes (Fig. 4B). Together with our light microscopy analysis of brain sections, this reveals that regions of severe neurodegeneration coincide with the regions of the CNS showing the most lysosomal storage.
4.5. dSap-rC27/Df mutant CNS soma are enlarged with storage
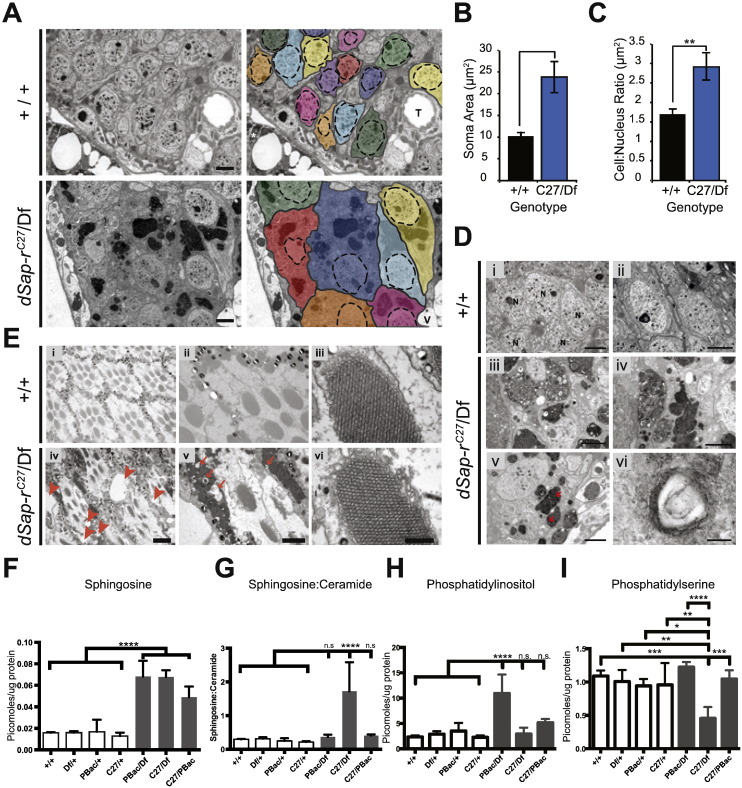
To investigate this lysosomal storage defect further, we performed transmission electron microscopy on 22-day old dSap-rC27/Df mutant antennal lobes, the same region where we saw the increased severity of neurodegeneration. We observed that dSap-rC27/Df mutant neuronal soma were consistently and grossly enlarged with electron-dense storage material. To quantify this difference in soma size, the boundaries of each neuron were marked using ImageJ and the soma area measured (Fig. 5A). Measuring the average soma area revealed an almost 2.5-fold increase in dSap-rC27/Df mutants compared to wild type (Fig. 5B). When normalised to nucleus area, the dSap-rC27/Df mutant soma were almost 2-fold larger, suggesting that nuclear area is also increased in dSap-rC27/Df mutant neurons (Fig. 5C). We also observed cell enlargement and increased storage in glial cells of the dSap-rC27/Df mutant adult CNS (Supplemental Fig. 2).
Fig. 5.
Neuronal swelling, degeneration and lipid storage are abundant in dSap-r mutant brains A. Transmission electron micrographs showing neuronal cell bodies adjacent to the antennal lobes of 22-day-old wild type (+/+) and dSap-rC27/Df mutants (n = 3). Each soma has been demarcated in grey and rendered a different pseudocolour. The nuclei are demarcated by dashed lines. T, trachea; V, vacuole; white asterisk, fat body. Scale bar: 2 μm. B & C. Quantification of 22-day-old wild type (+/+) and dSap-rC27/Df (C27/Df) mutant soma area (B) and cell:nucleus ratio (C). **p < 0.005. D. Transmission electron micrographs of 22-day-old wild type (+/+) and dSap-rC27/Df mutant neuronal cell bodies surrounding the antennal lobes (n = 3). Multivesicular bodies and multilamellar bodies (arrows) are abundant in dSap-rC27/Df mutant neuronal cell bodies. Scale bars: 2 μm (i–v), 100 nm (vi). E. Transmission electron micrographs showing the integrity of the ommatidia in 22-day-old wild type (+/+) and dSap-rC27/Df mutants. Arrowheads mark vacuoles and arrows mark electron lucent material within regions of electron-dense storage. Scale bars: 5 μm (i & iv), 2 μm (ii & v) and 500 nm (iii & vi). F—I. Quantification of sphingosine levels (F), the sphingosine:ceramide ratio (G), the phosphatidylinositol level (H), and phosphatidylserine (I) in 5-day-old controls and dSap-r mutant brains. Genotypes tested: +/+ (wild type); Df/+, PBac/+ and C27/+ (dSap-r heterozygotes); PBac/Df, C27/Df and C27/PBac (dSap-r mutants). *p < 0.05, **p < 0.01, ***p < 0.000, ****p < 0.0001 as determined by ANOVA followed by post-hoc Bonferonni test.
As a common characteristic of LSDs is the accumulation of membranous storage material known as multilamellar bodies (MLBs) and enlarged multivesicular bodies (MVBs), we investigated whether the 22-day old dSap-rC27/Df mutants showed these hallmark signs in the cortex surrounding the antennal lobes. Electron micrographs revealed neurons with an abundance of electron dense storage material that had a complex morphology; some regions were populated by electron-lucent droplets, some contained variable numbers of MLBs, and others were densely packed with MVBs (Fig. 5D). Electron micrographs also revealed an abundance of stored material in photoreceptor neurons of the fly retina (Fig. 5E), another sensory region of the nervous system that showed severe degeneration (Fig. 3A). This stored material had a similar morphology to that found in the antennal lobe neurons (Fig. 5D). High magnification images showed a variable phenotype in the rhabdomeres, the light responsive component of the eye. Most rhabdomeres showed massive accumulation of electron-dense material yet some were found to be structurally intact (Fig. 5E).
The ultrastructural nature of storage material in saposin deficiency patients and mammalian models has been shown to be highly variable, with the presence of MLBs, MVBs and electron-lucent droplets (Fujita et al., 1996, Harzer et al., 1989, Hulkova et al., 2001, Oya et al., 1998). The ultrastructural pathology observed in the dSap-r mutants is therefore consistent with the reported disease phenotype, which further supports the use of this model for investigating saposin deficiency.
4.6. Sphingolipids accumulate in dSap-r mutant brains
To gain a greater insight into the nature of the stored material in dSap-r mutants, we performed mass spectrometry. As saposin deficiency leads to the accumulation of a complex array of sphingolipids and sphingolipid intermediates, we determined the nature of sphingolipid perturbation in dSap-r mutants by monitoring Hexosyl-Ceramides (HexCer), Ceramide phosphorylethanolamine (CerPE), Ceramide (Cer) and Sphingosine (the breakdown product of Ceramide;(Yuan et al., 2011)) in conjunction with a lipidomic analysis of all major membrane phospholipids in the brain.
Brains from 5-day old flies revealed a consistent increase in sphingolipids, particularly sphingosine, in all dSap-r mutants examined (Fig. 5F). Notably, comparison of the sphingosine:ceramide ratios in controls and mutants revealed a striking imbalance between these sphingolipid intermediates in the dSap-rC27/Df mutants (Fig. 5G). We did not observe a significant change in HexCer across the mutant combinations.
Changes in particular phospholipid classes were observed. However, unlike the sphingolipids, there is no specific and consistent trend for changes in phospholipid classes across the different dSap-r mutant combinations tested (Fig. 5H&I).
4.7. dSap-r mutants show progressive physiological decline
After establishing that dSap-r mutants showed age-dependent neurodegeneration, we investigated the effect of dSap-r mutation on the physiology of the whole fly. We observed that the dSap-r mutant flies showed an age-dependent decline in locomotion; the 20 + day-old dSap-r mutants rarely climbed the vial during spontaneous activity. To quantify this loss of climbing behaviour, 5-day and 22-day-old female flies were tapped to the bottom of a measuring cylinder and their climbing response was captured by video for 45 s. Calculation of climbing speed revealed that the 22-day-old dSap-rC27/Df mutants were only able to climb at 5% of their 5-day-old speed compared to maintenance of 36% of 5-day climbing speed for the dSap- rC27/+ controls. The dSap-rC27/PBac mutants showed an intermediate phenotype maintaining 16% of their 5-day climbing ability at 22-days old. These data confirm an age-dependent deterioration of climbing behaviour in the dSap-r mutants (Fig. 6A).
The decline of locomotor ability in dSap-r mutants could be due to a deterioration of motor neuron or sensory neuron function; however, we have shown that sensory regions of the brain, including the visual system, are particularly vulnerable to dSap-r mutation. To determine whether the photoreceptor neurons and their underlying optic lobe synapses were functionally intact, we measured the ability of the fly eye to respond to light using the electroretinogram (ERG). The ERG is a classic method for investigating photoreceptor and lamina neuron function by measuring the summed potential difference on the surface of the fly eye in response to light pulses (Coombe, 1986, Heisenberg, 1971). Different components of the ERG trace represent the net influence of different parts of the visual system, therefore, the integrity of the different components of visual transduction can be assessed using this simple approach.
ERG recordings in 5-day-old dSap-rC27/Df mutants were not significantly different from controls in all components measured (Fig. 6B & C). However, 22-day-old dSap-rC27/Df mutants showed a severe deterioration of all components of the ERG compared to controls. The dSap-rC27/Df mutant ERG potential deteriorated to 46% of its 5-day value, whereas the dSap-rC27/+ control ERG potential only decreased to 77% of its 5-day value. This severe deterioration of the ERG amplitude in dSap-rC27/Df mutants was also matched by a 5-fold increase in recovery time after termination of the light pulse. This suggests that neuronal function in both the retina and underlying optic lobe is severely compromised in dSap-r mutants.
4.8. Calcium homeostasis is defective in dSap-r mutants
Calcium homeostasis defects have been highlighted as one aspect of cellular pathology implicated in the sphinoglipidoses (Ginzburg et al., 2004, Jeyakumar et al., 2005, Lloyd-Evans et al., 2008). For example, in NPC1 cells lysosomal calcium levels have been shown to be abnormally low, and by increasing cytosolic calcium levels NPC pathology in both NPC1 cells and mouse models can be substantially rescued (Lloyd-Evans et al., 2008). Calcium homeostasis defects have also been implicated in other sphingolipidoses (Gaucher's, Sandhoff and GM1-gangliosidosis); however, in these disorders an increase, rather than a decrease, in cytosolic calcium levels was found to be pathological (Lloyd-Evans et al., 2003a, Lloyd-Evans et al., 2003b, Pelled et al., 2003, Pelled et al., 2005). This suggests that both abnormally low or abnormally high levels of calcium might be implicated in Sphingolipidoses. Furthermore, calcium homeostasis defects have been mechanistically linked to an imbalance in sphingolipid levels in a fly model of retinal degeneration (Yonamine et al., 2011). Based on our findings of an imbalance of sphingolipids prior to severe signs of neurodegeneration in the dSap-r mutants, we hypothesised that calcium levels may also be altered.
To investigate calcium misregulation and its impact on dSap-r neurodegeneration, we used a similar approach to Yonamine et al. (2011) and manipulated calcium levels in the fly eye. We overexpressed the plasma membrane calcium exchanger (CalX) in the fly eye using the NINAE (Rhodopsin 1) promoter and measured its effect on retinal function using ERGs. CalX transports calcium ions out of the neuron in exchange for sodium ions (Wang et al., 2005). Therefore, if dSap-r mutants have a reduced cytosolic calcium level, CalX expression should exacerbate the degeneration and function of the photoreceptors. In contrast, the ERG should be substantially rescued if dSap-r mutation causes an increased cytosolic calcium level.
Overexpression of CalX in an otherwise wild type fly resulted in a modest degeneration of the ERG to 81% of its 5-day level (Fig. 6D & E). When CalX was expressed in a dSap-rC27/Df mutant background, there was no effect on the 5-day-old dSap-rC27/Df mutant ERG. However, as the flies aged, CalX expression induced a decrease in dSap-rC27/Df mutant vision to only 9% of its 5-day-old level; this is in contrast to the deterioration of the dSap-rC27/Df mutant ERG to 64% of its 5-day-old level, and therefore suggests that the dSap-r mutation may cause an abnormally reduced cytosolic calcium level.
5. Discussion
To further our understanding of the pathology related to saposin deficiency, we generated a Drosophila model. The Drosophila prosaposin homologue dSap-r was revealed to contain eight saposin-like domains, which all contained the six-cysteine primary sequence common to all saposins (this investigation, (Hazkani-Covo et al., 2002)). We showed that most of the dSap peptides contain a predicted glycosylation site, a feature critical for saposin folding and function (Hiraiwa et al., 1993b). The sequence similarities between the mammalian and Drosophila saposins suggested that we had identified the correct ortholog.
To further characterise dSap-r, we investigated its expression pattern using GFP reporters. Both membrane and nuclear GFP reporters confirmed the expression of dSap-r in glia, as reported by (Freeman et al., 2003). Although mammalian saposins are expressed in neurons, our findings suggested that dSap-r is either not expressed in neurons or is expressed at a level below the threshold of our reporter. Transcriptomic data from FACS isolated neurons, glia and surface glia (blood-brain barrier) (DeSalvo et al., 2014) shows that dSap-r is expressed in neurons, glia and surface glia, and may therefore be more ubiquitous than the dSap-rNP7456 GAL4 enhancer-trap is reporting.
In addition, mammalian prosaposin is abundant in secretory fluids, including cerebrospinal fluid, seminal fluid, milk, bile and pancreatic fluid (Hineno et al., 1991, Hiraiwa et al., 1993a, Kondoh et al., 1991, Patton et al., 1997); in fact prosaposin is one of the main secretory products of Sertoli cells in the male reproductive system (Sylvester et al., 1989). Therefore, it is quite possible that Drosophila glia provide dSap-r to neurons by glial secretion and neuronal uptake. This notion is consistent with our findings that dSap-r mutant longevity can be substantially rescued by expressing dSap-r directly in neurons, which suggests a neuronal requirement for dSap-r. This potential non-autonomous function of dSap-r has important implications for future therapeutic strategies as it suggests that a source of prosaposin secreting cells could provide a successful intervention for these conditions.
This investigation also revealed high dSap-r expression in the male and female reproductive organs, the digestive system, Drosophila renal system (Malpighian tubules), and the Drosophila liver equivalent (the fat bodies). Mammalian prosaposin has a role in spermiogenesis and improving fertility (Amann et al., 1999, Hammerstedt, 1997, Morales et al., 2000, Sun et al., 2007), and is also relatively abundant in subsets of cells of the small intestine, kidney and liver (Sun et al., 1994). Therefore, in addition to having a conserved primary sequence, dSap-r also has a conserved tissue expression suggesting a conserved function.
After confirming the identity of the Drosophila prosaposin orthologue and its expression pattern, we generated a dSap-r loss-of-function model and assessed its pathology. Ultrastructural analysis revealed extensive storage in the dSap-r mutant nervous system (MLBs, enlarged MVBs and lipid droplets), characteristic of LSD pathology. Lysosomal storage was also confirmed by western blotting, which showed progressive accumulation of two lysosomal markers (Arl8 and cathepsin-L) in the dSap-r mutants, and mass spectrometry revealed early accumulation and imbalance of sphingolipid intermediates in dSap-r mutants.
The dSap-rC27/Df mutants were the most severe mutants in all assays tested. They also showed the most severe imbalance of sphingosine:ceramide, whilst having similar levels of sphingosine and lower levels of ceramide than the other mutant allelic combinations. This therefore supports previous findings that the ratio of sphingolipids is more important than the total levels of individual sphingolipids when considering the effect on pathology (Fewou et al., 2005, Levade et al., 1995, Matsuda et al., 2004, Sun et al., 2008, Sun et al., 2007).
In prosaposin and individual saposin deficiency mouse models, the stored material was described as very heterogeneous with electron-dense and electron-lucent inclusions, MLBs and MVBs (Matsuda et al., 2004, Matsuda et al., 2001, Oya et al., 1998, Sun et al., 2010a, Sun et al., 2010b, Sun et al., 2008, Sun et al., 2007). This phenotype is strikingly similar to the ultrastructural pathology of dSap-r mutants.
Like other Drosophila LSD models, dSap-r mutants die prematurely, with 50% death within 18 days; this equates to a 41–49% reduction in longevity, which is comparable to most Drosophila LSD models (40–55% reduction) (Hickey et al., 2006, Huang et al., 2007, Min and Benzer, 1997, Nakano et al., 2001, Phillips et al., 2008). The dSap-r mutants also showed an age-dependent deterioration of spontaneous and evoked locomotion, which correlated with the progressive nature of vacuolarisation in the sensory regions of the dSap-r mutant nervous system. This may suggest that locomotion deterioration is primarily a result of failed perception of sensory cues rather than motor neuron or muscle degeneration. This notion is supported by a less severe deterioration in dSap-r mutant jump performance using an assay that bypasses sensory neuron input (data not shown). A similar degeneration in sensory function is observed in both Drosophila and mouse models of NPC1 (Claudepierre et al., 2010, Palladino et al., 2015, Phillips et al., 2008, Yan et al., 2014), and recently recognised in NPC1 patients (Zafeiriou et al., 2003). These results suggest a particular sensitivity of sensory neurons to disruptions in sphingolipid metabolism. This is also supported by the severe sensory degeneration observed in the sphingolipid synthesis disorder Hereditary Sensory and Autonomic Neuropathy Type 1 (HSAN1) (Dawkins et al., 2001). HSAN1 is caused by mutations in the first enzyme of the sphingolipid synthesis pathway: serine-palmitoyl transferase (SPT). Although HSAN1 is a sphingolipid synthesis disorder and saposin deficiency is a disorder of sphingolipid degradation, the sensory degeneration in both of these disorders reflects a common cellular pathology: a defect in sphingolipid homeostasis. Therefore, sensory degeneration may result from an absence of sphingolipids, an abnormal accumulation of sphingolipids, an imbalance of sphingolipids, or a combination of all three.
Although retinal degeneration and storage has been reported in other Drosophila models of LSDs (Dermaut et al., 2005, Myllykangas et al., 2005, Phillips et al., 2008, Tuxworth et al., 2009), the pathological mechanism remains unclear. In several mammalian sphingolipidoses models, recent reports have revealed calcium homeostasis defects. Therefore, a calcium exchanger was overexpressed in the fly eye of dSap-r mutants to determine whether altering cytosolic calcium levels had any effect on dSap-r mutant retinal function. Aging of dSap-rC27/Df mutants caused a 36% reduction in retinal function; a similar but less severe (19%, n.s.) deterioration was also seen after expression of CalX in aged wild type flies. Overexpression of CalX in a dSap-r mutant background caused a severe (92%) deterioration of retinal function, leaving the 22-day-old flies almost completely unresponsive to light. This significant genetic interaction suggests that calcium regulation may be compromised in dSap-r mutant photoreceptors, leading to neurodegeneration and perturbed sensory function. Further work is required to determine the severity of the calcium homeostasis defect in dSap-r mutants, including its temporal and spatial (neurons vs glia) involvement.
The Drosophila NPC1 model revealed degeneration of the visual system and concomitant deterioration of the ERG that resembles that of the dSap-r mutant model (Phillips et al., 2008). Although classically characterised as a cholesterol-storage disorder, mammalian NPC pathology has more recently been linked to sphingolipid storage (Lloyd-Evans et al., 2008), specifically sphingosine storage leading to a deficit in lysosomal calcium regulation (Lloyd-Evans et al., 2008). In support of this, in cell culture, NPC1 cells contained approximately 65% less lysosomal calcium compared to control cells. It was shown that the sphingolipid intermediate sphingosine was responsible for this calcium homeostasis defect (Lloyd-Evans et al., 2008), directly linking calcium homeostasis defects to the accumulation of sphingolipids in the sphingolipidoses.
Our sphingolipid analyses showed that sphingosine levels were significantly increased in all dSap-r mutant brains. Like in NPC1 cells, this may cause a decrease in lysosomal calcium in dSap-r mutants, which was further exacerbated by overexpression of CalX. Further evidence of a link between sphingolipid metabolism and calcium homeostasis was also provided by Yonamine et al. (2011), who revealed that retinal degeneration caused by imbalances in sphingolipid metabolites was suppressed by mutations in the Drosophila CalX. Similar to Yonamine et al. (2011) we have also shown an imbalance in the ratio between two sphingolipid intermediates in dSap-r mutant brains, providing further support of calcium homeostasis defects being a likely cause of dSap-r mutant pathology.
In conclusion, we suggest that early accumulation of sphingosine, or the imbalance of the sphingosine:ceramide ratio, may lead to a decrease in lysosomal calcium and degeneration of sensory neurons in dSap-r mutants, which leads to physiological deterioration. Therapeutic interventions to increase cytosolic calcium levels in saposin-deficient patients may provide a successful route for ameliorating these disorders.
The following are the supplementary data related to this article.
dSap-r is expressed in visceral organs of Drosophila. Digestive systems (A), male (B) and female (D) reproductive systems and fat bodies (C) are shown from adult controls (+/+) and flies expressing mCD8eGFP under the control of dSap-rNP7456 GAL4. Organs are stained with the nuclear marker DAPI in (A). MT, Malpighian tubule; Mg, midgut; Hg, hindgut; T, testes; EB, ejaculatory bulb; AG, accessory gland; ED, ejaculatory duct; O, ovary; S, spermatheca. Scale bars: (A) 1000 μm (vii) and 250 μm (viii), (B-D) 500 μm.
Cell enlargement and increased storage in dSap-rC27/Df mutant glia. Transmission electron micrographs of glia surrounding the antennal lobe of 22-day old wild type (+/+; A) and dSap-rC27/Df mutant (B) brains (n = 3). Electron-dense and electron-lucent vesicular storage is shown in dSap-rC27/Df mutant glia (B). Scale bar: 1 μm.
Internal Standard (IS) mix used for lipidomics
Funding
This work was funded by a Quota studentship from the BBSRC [to S.J.H] (BB/D527026/1) and a Medical Research Council grant [G0400580 to S.T.S]. Work in the D.S laboratory was supported by a Senior Fellowship of the Wellcome Trust/DBT India Alliance, and the NCBS-Merck & Co International Investigator Award [D.S].
Conflict of interest statement
None declared.
Acknowledgements
For fly stocks and reagents we would like to thank Andreas Prokop, Debbie Smith, Dani Ungar, Roger Hardie, The Bloomington and Kyoto Drosophila stock centres and the Developmental Studies Hybridoma Bank, Iowa. The anti-repo, anti-elav and anti-tubulin monoclonal antibodies, developed by Corey Goodman, Gerald M. Rubin and Michael Klymkowsky, were obtained from the DSHB, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa, IA 52242. We would like to thank Gareth Evans for invaluable help with densitometry and experimental guidance. For technical advice we are indebted to Meg Stark, Karen Hodgkinson, Graeme Park and Paul Pryor.
References
- Amann R.P. Exposure of human, boar, or bull sperm to a synthetic peptide increases binding to an egg-membrane substrate. J. Androl. 1999;20:34–41. [PubMed] [Google Scholar]
- Azuma N. Stimulation of acid ceramidase activity by saposin D. Arch. Biochem. Biophys. 1994;311:354–357. doi: 10.1006/abbi.1994.1248. [DOI] [PubMed] [Google Scholar]
- Berent S.L., Radin N.S. Mechanism of activation of glucocerebrosidase by co-beta-glucosidase (glucosidase activator protein) Biochim. Biophys. Acta. 1981;664:572–582. doi: 10.1016/0005-2760(81)90134-x. [DOI] [PubMed] [Google Scholar]
- Christomanou H. Immunochemical characterization of two activator proteins stimulating enzymic sphingomyelin degradation in vitro. Absence of one of them in a human Gaucher disease variant. Biol. Chem. Hoppe Seyler. 1986;367:879–890. doi: 10.1515/bchm3.1986.367.2.879. [DOI] [PubMed] [Google Scholar]
- Claudepierre T. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Mol. Cell. Neurosci. 2010;43:164–176. doi: 10.1016/j.mcn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Coombe P.E. The large monopolar cells L1 and L2 are responsible for the ERG transients in Drosophila. J. Comp. Physiol. 1986;159:655–666. [Google Scholar]
- Dawkins J.L. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat. Genet. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- Dermaut B. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J. Cell Biol. 2005;170:127–139. doi: 10.1083/jcb.200412001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo M.K. The Drosophila surface glia transcriptome: evolutionary conserved blood-brain barrier processes. Front. Neurosci. 2014;8:346. doi: 10.3389/fnins.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleder M. Niemann-Pick disease type C with enhanced glycolipid storage. Report on further case of so-called lactosylceramidosis. Virchows Arch. A Pathol. Anat. Histopathol. 1984;402:307–317. doi: 10.1007/BF00695084. [DOI] [PubMed] [Google Scholar]
- Erickson A.H. Biosynthesis of lysosomal endopeptidases. J. Cell. Biochem. 1989;40:31–41. doi: 10.1002/jcb.240400104. [DOI] [PubMed] [Google Scholar]
- Fewou S.N. Reversal of non-hydroxy:alpha-hydroxy galactosylceramide ratio and unstable myelin in transgenic mice overexpressing UDP-galactose:ceramide galactosyltransferase. J. Neurochem. 2005;94:469–481. doi: 10.1111/j.1471-4159.2005.03221.x. [DOI] [PubMed] [Google Scholar]
- Fluegel M.L. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics. 2006;172:185–196. doi: 10.1534/genetics.105.046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.R. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Fujita N. Targeted disruption of the mouse sphingolipid activator protein gene: a complex phenotype, including severe leukodystrophy and wide-spread storage of multiple sphingolipids. Hum. Mol. Genet. 1996;5:711–725. doi: 10.1093/hmg/5.6.711. [DOI] [PubMed] [Google Scholar]
- Furst W. The precursor of sulfatide activator protein is processed to three different proteins. Biol. Chem. Hoppe Seyler. 1988;369:317–328. doi: 10.1515/bchm3.1988.369.1.317. [DOI] [PubMed] [Google Scholar]
- Ginzburg L. The pathogenesis of glycosphingolipid storage disorders. Semin. Cell Dev. Biol. 2004;15:417–431. doi: 10.1016/j.semcdb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hammerstedt, R. H., 1997. A method and use of polypeptide in sperm-egg binding to enhance or decrease fertility. International Patent Publication Number W)/97/25620 Geneva: World International Property Organization. 1–42.
- Harzer K. Sphingolipid activator protein deficiency in a 16-week-old atypical Gaucher disease patient and his fetal sibling: biochemical signs of combined sphingolipidoses. Eur. J. Pediatr. 1989;149:31–39. doi: 10.1007/BF02024331. [DOI] [PubMed] [Google Scholar]
- Hazkani-Covo E. The evolutionary history of prosaposin: two successive tandem-duplication events gave rise to the four saposin domains in vertebrates. J. Mol. Evol. 2002;54:30–34. doi: 10.1007/s00239-001-0014-0. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Separation of receptor and lamina potentials in the electroretinogram of normal and mutant Drosophila. J. Exp. Biol. 1971;55:85–100. doi: 10.1242/jeb.55.1.85. [DOI] [PubMed] [Google Scholar]
- Herzog R. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011;12:R8. doi: 10.1186/gb-2011-12-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey A.J. Palmitoyl-protein thioesterase 1 deficiency in Drosophila melanogaster causes accumulation of abnormal storage material and reduced life span. Genetics. 2006;172:2379–2390. doi: 10.1534/genetics.105.053306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle S. Dopaminergic expression of the Parkinsonian gene LRRK2-G2019S leads to non-autonomous visual neurodegeneration, accelerated by increased neural demands for energy. Hum. Mol. Genet. 2013;22:2129–2140. doi: 10.1093/hmg/ddt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hineno T. Secretion of sphingolipid hydrolase activator precursor, prosaposin. Biochem. Biophys. Res. Commun. 1991;176:668–674. doi: 10.1016/s0006-291x(05)80236-0. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M. Isolation, characterization, and proteolysis of human prosaposin, the precursor of saposins (sphingolipid activator proteins) Arch. Biochem. Biophys. 1993;304:110–116. doi: 10.1006/abbi.1993.1328. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M. The effect of carbohydrate removal on stability and activity of saposin B. Arch. Biochem. Biophys. 1993;303:326–331. doi: 10.1006/abbi.1993.1291. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M. Lysosomal proteolysis of prosaposin, the precursor of saposins (sphingolipid activator proteins): its mechanism and inhibition by ganglioside. Arch. Biochem. Biophys. 1997;341:17–24. doi: 10.1006/abbi.1997.9958. [DOI] [PubMed] [Google Scholar]
- Hofmann I., Munro S. An N-terminally acetylated Arf-like GTPase is localised to lysosomes and affects their motility. J. Cell Sci. 2006;119:1494–1503. doi: 10.1242/jcs.02958. [DOI] [PubMed] [Google Scholar]
- Huang X. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development. 2005;132:5115–5124. doi: 10.1242/dev.02079. [DOI] [PubMed] [Google Scholar]
- Huang X. Drosophila Niemann-Pick type C-2 genes control sterol homeostasis and steroid biosynthesis: a model of human neurodegenerative disease. Development. 2007;134:3733–3742. doi: 10.1242/dev.004572. [DOI] [PubMed] [Google Scholar]
- Hulkova H. A novel mutation in the coding region of the prosaposin gene leads to a complete deficiency of prosaposin and saposins, and is associated with a complex sphingolipidosis dominated by lactosylceramide accumulation. Hum. Mol. Genet. 2001;10:927–940. doi: 10.1093/hmg/10.9.927. [DOI] [PubMed] [Google Scholar]
- Jardim L.B. Clinical aspects of neuropathic lysosomal storage disorders. J. Inherit. Metab. Dis. 2010;33:315–329. doi: 10.1007/s10545-010-9079-5. [DOI] [PubMed] [Google Scholar]
- Jeyakumar M. Storage solutions: treating lysosomal disorders of the brain. Nat. Rev. Neurosci. 2005;6:713–725. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- Kondoh K. Isolation and characterization of prosaposin from human milk. Biochem. Biophys. Res. Commun. 1991;181:286–292. doi: 10.1016/s0006-291x(05)81415-9. [DOI] [PubMed] [Google Scholar]
- Levade T. Neurodegenerative course in ceramidase deficiency (Farber disease) correlates with the residual lysosomal ceramide turnover in cultured living patient cells. J. Neurol. Sci. 1995;134:108–114. doi: 10.1016/0022-510x(95)00231-0. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J. Biol. Chem. 2003;278:23594–23599. doi: 10.1074/jbc.M300212200. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E. Lyso-glycosphingolipids mobilize calcium from brain microsomes via multiple mechanisms. Biochem. J. 2003;375:561–565. doi: 10.1042/BJ20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- Matsuda J. A mutation in the saposin A domain of the sphingolipid activator protein (prosaposin) gene results in a late-onset, chronic form of globoid cell leukodystrophy in the mouse. Hum. Mol. Genet. 2001;10:1191–1199. doi: 10.1093/hmg/10.11.1191. [DOI] [PubMed] [Google Scholar]
- Matsuda J. Mutation in saposin D domain of sphingolipid activator protein gene causes urinary system defects and cerebellar Purkinje cell degeneration with accumulation of hydroxy fatty acid-containing ceramide in mouse. Hum. Mol. Genet. 2004;13:2709–2723. doi: 10.1093/hmg/ddh281. [DOI] [PubMed] [Google Scholar]
- Matyash V. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K.T., Benzer S. Spongecake and eggroll: two hereditary diseases in Drosophila resemble patterns of human brain degeneration. Curr. Biol. 1997;7:885–888. doi: 10.1016/s0960-9822(06)00378-2. [DOI] [PubMed] [Google Scholar]
- Morales C.R. Targeted disruption of the mouse prosaposin gene affects the development of the prostate gland and other male reproductive organs. J. Androl. 2000;21:765–775. [PubMed] [Google Scholar]
- Morimoto S. Saposin A: second cerebrosidase activator protein. Proc. Natl. Acad. Sci. U. S. A. 1989;86:3389–3393. doi: 10.1073/pnas.86.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllykangas L. Cathepsin D-deficient Drosophila recapitulate the key features of neuronal ceroid lipofuscinoses. Neurobiol. Dis. 2005;19:194–199. doi: 10.1016/j.nbd.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Nakano T. Structure of full-length cDNA coding for sulfatide activator, a Co-beta-glucosidase and two other homologous proteins: two alternate forms of the sulfatide activator. J. Biochem. 1989;105:152–154. doi: 10.1093/oxfordjournals.jbchem.a122629. [DOI] [PubMed] [Google Scholar]
- Nakano Y. Mutations in the novel membrane protein spinster interfere with programmed cell death and cause neural degeneration in Drosophila melanogaster. Mol. Cell. Biol. 2001;21:3775–3788. doi: 10.1128/MCB.21.11.3775-3788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J.S., Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 1991;5:301–308. doi: 10.1096/fasebj.5.3.2001789. [DOI] [PubMed] [Google Scholar]
- O'Brien J.S. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988;241:1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- Oya Y. Pathological study of mice with total deficiency of sphingolipid activator proteins (SAP knockout mice) Acta Neuropathol. 1998;96:29–40. doi: 10.1007/s004010050857. [DOI] [PubMed] [Google Scholar]
- Palladino G. Visual evoked potentials of Niemann-Pick type C1 mice reveal an impairment of the visual pathway that is rescued by 2-hydroxypropyl-ss-cyclodextrin. Orphanet J. Rare Dis. 2015;10:133. doi: 10.1186/s13023-015-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S. Prosaposin, a neurotrophic factor: presence and properties in milk. J. Dairy Sci. 1997;80:264–272. doi: 10.3168/jds.S0022-0302(97)75934-4. [DOI] [PubMed] [Google Scholar]
- Pelled D. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2 +-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J. Biol. Chem. 2003;278:29496–29501. doi: 10.1074/jbc.M302964200. [DOI] [PubMed] [Google Scholar]
- Pelled D. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol. Dis. 2005;18:83–88. doi: 10.1016/j.nbd.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillips S.E. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J. Neurosci. 2008;28:6569–6582. doi: 10.1523/JNEUROSCI.5529-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwudke D. Shotgun lipidomics on high resolution mass spectrometers. Cold Spring Harb. Perspect. Biol. 2011;3:a004614. doi: 10.1101/cshperspect.a004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R. A mutation in the saposin A coding region of the prosaposin gene in an infant presenting as Krabbe disease: first report of saposin A deficiency in humans. Mol. Genet. Metab. 2005;84:160–166. doi: 10.1016/j.ymgme.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Spurr A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sun Y. Developmental and tissue-specific expression of prosaposin mRNA in murine tissues. Am. J. Pathol. 1994;145:1390–1398. [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Combined saposin C and D deficiencies in mice lead to a neuronopathic phenotype, glucosylceramide and alpha-hydroxy ceramide accumulation, and altered prosaposin trafficking. Hum. Mol. Genet. 2007;16:957–971. doi: 10.1093/hmg/ddm040. [DOI] [PubMed] [Google Scholar]
- Sun Y. Neurological deficits and glycosphingolipid accumulation in saposin B deficient mice. Hum. Mol. Genet. 2008;17:2345–2356. doi: 10.1093/hmg/ddn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Neuronopathic Gaucher disease in the mouse: viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum. Mol. Genet. 2010;19:1088–1097. doi: 10.1093/hmg/ddp580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Specific saposin C deficiency: CNS impairment and acid beta-glucosidase effects in the mouse. Hum. Mol. Genet. 2010;19:634–647. doi: 10.1093/hmg/ddp531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester S.R. Sulfated glycoprotein-1 (saposin precursor) in the reproductive tract of the male rat. Biol. Reprod. 1989;41:941–948. doi: 10.1095/biolreprod41.5.941. [DOI] [PubMed] [Google Scholar]
- Tuxworth R.I. Interactions between the juvenile Batten disease gene, CLN3, and the Notch and JNK signalling pathways. Hum. Mol. Genet. 2009;18:667–678. doi: 10.1093/hmg/ddn396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylki-Szymanska A. Non-neuronopathic Gaucher disease due to saposin C deficiency. Clin. Genet. 2007;72:538–542. doi: 10.1111/j.1399-0004.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe L. Prosaposin gene expression in normal and dystrophic RCS rat retina. Invest. Ophthalmol. Vis. Sci. 2004;45:1297–1305. doi: 10.1167/iovs.03-1048. [DOI] [PubMed] [Google Scholar]
- Vogel A. Identity of the activator proteins for the enzymatic hydrolysis of sulfatide, ganglioside GM1, and globotriaosylceramide. Arch. Biochem. Biophys. 1987;259:627–638. doi: 10.1016/0003-9861(87)90529-7. [DOI] [PubMed] [Google Scholar]
- Wang T. Light activation, adaptation, and cell survival functions of the Na+/Ca2 + exchanger CalX. Neuron. 2005;45:367–378. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Yamada M. Analysis of recombinant human saposin A expressed by Pichia pastoris. Biochem. Biophys. Res. Commun. 2004;318:588–593. doi: 10.1016/j.bbrc.2004.04.069. [DOI] [PubMed] [Google Scholar]
- Yan X. Defects in the retina of Niemann-Pick type C1 mutant mice. BMC Neurosci. 2014;15:126. doi: 10.1186/s12868-014-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonamine I. Sphingosine kinases and their metabolites modulate endolysosomal trafficking in photoreceptors. J. Cell Biol. 2011;192:557–567. doi: 10.1083/jcb.201004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshige A. Regional expression of prosaposin in the wild-type and saposin D-deficient mouse brain detected by an anti-mouse prosaposin-specific antibody. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009;85:422–434. doi: 10.2183/pjab.85.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C. CDase is a pan-ceramidase in Drosophila. Mol. Biol. Cell. 2011;22:33–43. doi: 10.1091/mbc.E10-05-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafeiriou D.I. Niemann-Pick type C disease associated with peripheral neuropathy. Pediatr. Neurol. 2003;29:242–244. doi: 10.1016/s0887-8994(03)00219-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
dSap-r is expressed in visceral organs of Drosophila. Digestive systems (A), male (B) and female (D) reproductive systems and fat bodies (C) are shown from adult controls (+/+) and flies expressing mCD8eGFP under the control of dSap-rNP7456 GAL4. Organs are stained with the nuclear marker DAPI in (A). MT, Malpighian tubule; Mg, midgut; Hg, hindgut; T, testes; EB, ejaculatory bulb; AG, accessory gland; ED, ejaculatory duct; O, ovary; S, spermatheca. Scale bars: (A) 1000 μm (vii) and 250 μm (viii), (B-D) 500 μm.
Cell enlargement and increased storage in dSap-rC27/Df mutant glia. Transmission electron micrographs of glia surrounding the antennal lobe of 22-day old wild type (+/+; A) and dSap-rC27/Df mutant (B) brains (n = 3). Electron-dense and electron-lucent vesicular storage is shown in dSap-rC27/Df mutant glia (B). Scale bar: 1 μm.
Internal Standard (IS) mix used for lipidomics