Fig. 2.
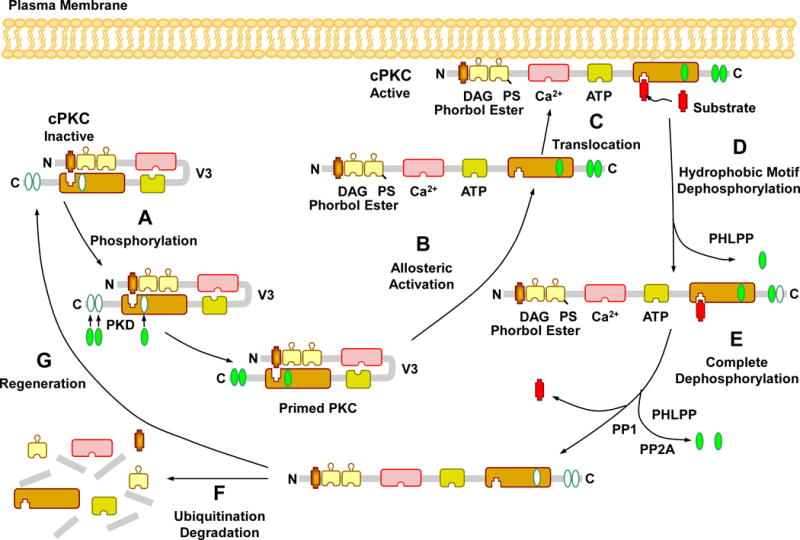
Activation, translocation, substrate interaction and deactivation of cPKCs. In the PKC cytosolic and inactive state, the pseudosubstrate binds the catalytic site in the C4 region, leading to folding of the regulatory and catalytic domain. Before it becomes catalytically competent, nascent PKC undergoes phosphorylation at three phosphorylation sites. The first and rate-limiting phosphorylation of the activation loop is catalyzed by phosphoinositide-dependent kinase (PDK). Consequently, a negative charge is introduced that properly aligns residues to form a competent catalytic domain and facilitate subsequent autophosphorylation at the turn motif and hydrophobic motif, a process that keeps PKC in a catalytically competent and protease resistant conformation. PKC activators such as PS, DAG, phorbol esters, and Ca2+ promote full allosteric activation and translocation of PKC to the plasma membrane. Allosteric activation also induces an open conformation state, making PKC susceptible to phosphatases and proteases and allows PKC to either enter an autophosphorylation/dephosphorylation cycle, or undergoes proteolytic degradation, PKC dephosphorylation terminates its kinase activity and is carried out by the PP2C member pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP) at the hydrophobic motif, starting the process that consequently drives complete dephosphorylation of PKC by PP1/PP2A protein phosphatases at the turn motif. Dephosphorylation also predisposes “naked” PKC to ubiquitination and degradation, requiring new synthesis of the enzyme.
