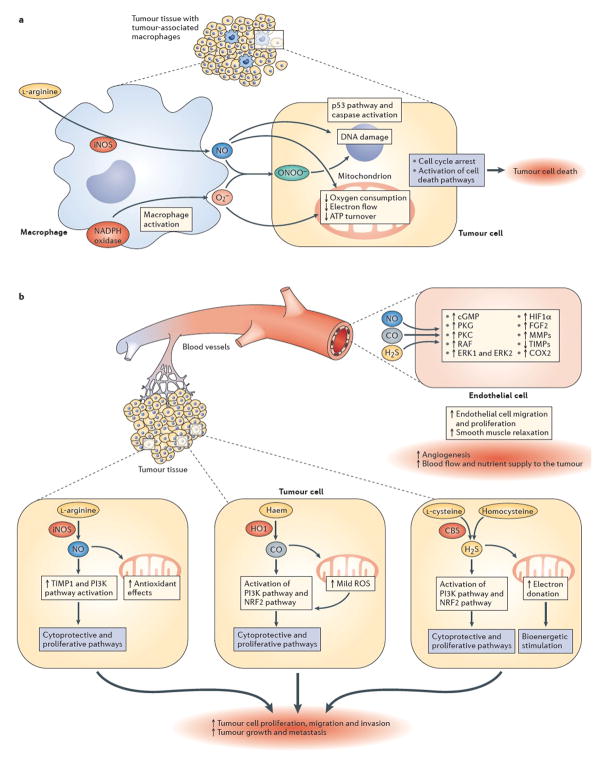
Figure 1. Effects of NO, CO and H2S on tumour survival and growth.
[a | NO-mediated mechanisms of tumour cell killing by tumour-associated macrophages. Upregulation of inducible nitric oxide synthase (iNOS) in activated tumour-associated macrophages leads to the production of high local levels of NO. At the same time, macrophages also produce superoxide (O2–) from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and other cellular sources. Together, NO and superoxide form peroxynitrite (ONOO–), a reactive oxidant species. The resulting combination of nitrosative and oxidative stress can be cytostatic or cytotoxic to certain tumour cell types (the NO-associated component of cell killing is tumour-cell-type dependent). In susceptible tumour cells, the NO-mediated cell killing involves the inhibition of mitochondrial activity, DNA damage and activation of downstream pathways including such as the p53 and caspase activation pathways, culminating in tumour cell lysis. These mechanisms can be enhanced by various immunostimulatory therapies and/or by supplementation of L-arginine, the substrate of NOS. b | Pro-tumour effects of low levels of endogenously produced NO, carbon monoxide (CO) and hydrogen sulfide (H2S). Survival and proliferation of the tumour cell is stimulated by gasotransmitter production within the tumour. Within the tumour, induction of iNOS and consequently elevated levels of NO (top right), induction of haem oxygenase 1 (HO1) and elevated levels of CO (middle cancer cell on top of the right side of the graph) and/or induction of cystathionine-β-synthase (CBS) and elevated levels of H2S (bottom right) can exert pro-survival and pro-proliferative effects. Depending on the gasotransmitter, these signalling mechanisms can culminate in upregulation of fibroblast growth factor 2 (FGF2), activation of matrix metalloproteinases (MMPs), upregulation of tissue inhibitors of matrix metalloproteinases (TIMPs), activation of PI3K, and/or the stimulation of the inducible isoform of cyclooxygenase (COX2). In addition to tumour-autonomous effects, each gasotransmitter can diffuse out from the tumour cells and can stimulate intra- and peritumour angiogenesis through paracrine actions on endothelial cells, for instance by stimulating various pro-angiogenic pathways (including the cyclic GMP–protein kinase G (PKG) signalling pathway, activation of protein kinase C (PKC), RAF, extracellular signal-regulated kinase 1 (ERK1) and ERK2, and stabilization of hypoxia inducible factor 1α (HIF1α)). Although the signalling mechanisms are gasotransmitter- and condition-dependent, the ultimate result is the stimulation of peritumour angiogenesis and an increase in tumour blood flow.