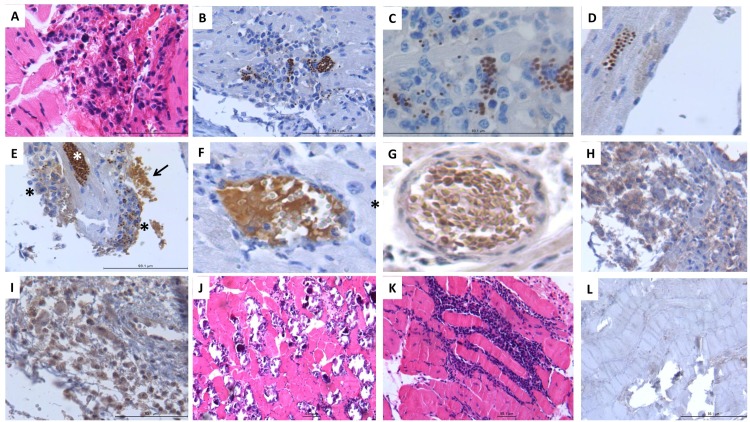
Fig 5. Histology and immunohistochemistry analysis of an experimental Chagas mouse model suggests that tissue-released TcHMGB can induce inflammatory cytokines production during acute infection.
A) Myocardial nodule constituted by macrophages and lymphocytes in acute infection. B) These inflammatory nodules show numerous spherules with strong TcHMGB immunostaining, which correspond to T. cruzi amastigotes. C) High power micrograph show T. cruzi amastigotes immunoreactive to TcHMGB in the cytoplasm of macrophages. D) Cardiomyocytes also show in the cytoplasm numerous T. cruzi amastigotes with strong immunereactivity to TcHMGB. E) Acute myocardiopathy show heavily infected myocytes with numerous parasites showing strong immunostaining to TcHMGB (white asterisk), the endocardium shows numerous attached macrophages with phagocytosed immunostained parasites or diffuse cytoplasmic immunostaining to TcHMGB (black asterisks), fibrillar and granular material that apparently correspond to fibrin exhibited strong TcHMGB immunostaining (arrow). F) Middle size blood vessels in the myocardium show intravascular fibrin that exhibited strong TcHMGB immunostaining. G) Similar myocardial blood vessels from acute chagasic myocarditis show leukocytes with immunostaining to TNFα. H) Acute inflammatory nodules show macrophages with immunoreactivity to IL-1β. I) Numerous perivascular inflammatory cells in acute trypanosomal myocarditis show immunoreactivity to IFN-γ. J) Necrotic myocytes, fibrosis and calcification in chronic chagasic cardiopathy with extensive chronic inflammatory infiltrate (K), without TcHMGB immunostaining (L).