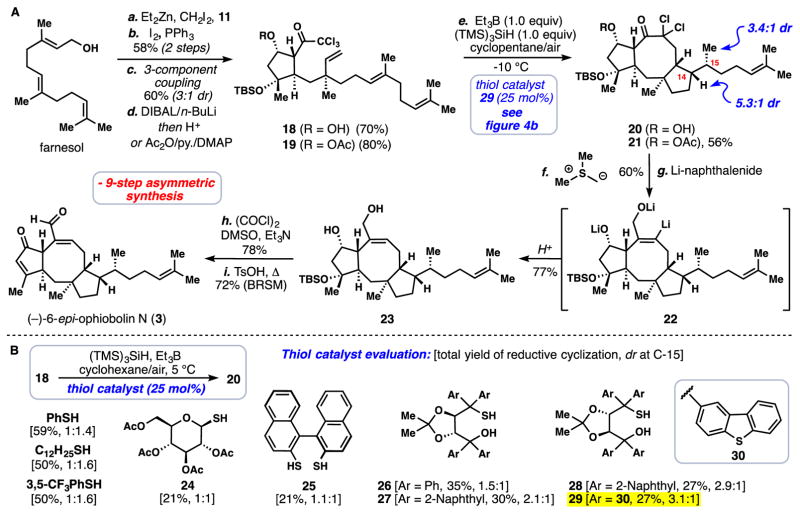
Fig. 4. Total synthesis of an ophiobolin sesterterpene.
(A) Nine-step asymmetric synthesis of (−)-6-epi-ophiobolin N (3) (yields reported for synthetic steps e-h are for the diastereomeric mixture) (B) Evaluation of thiol catalysts for the transformation of 18→20 (yields and selectivity determined by 1H NMR analysis, dr at C-14 was approximately 4:1). Reagents and conditions: (a-d) see Figure 3 for analogous conditions, (e) 19 (1.0 equiv), TMS3SiH (1.0 equiv), 29 (25 mol%), Et3B (1.0 M solution in THF, 1.25 equiv) added over 12 hours, air, cyclopentane (0.009 M), −10 °C, 12 hours, 56% combined yield of reductively cyclized material (the reported dr values at C-14 (5.3:1) and C-15 (3.4:1) were determined after synthetic step h (see SM); (f) Me3SI (24.0 equiv), n-BuLi (6.0 equiv), THF, 0 °C, 15 min, then add 21 (1.0 equiv), 10 min, 60%; (g) Lithium naphthalenide (1.0 M solution in THF, 40 equiv.), THF, −78 °C, 20 min, 77%; (h) (COCl)2 (10.0 equiv), DMSO (15.0 equiv), Et3N (20.0 equiv), CH2Cl2, −78 °C→0 °C, 3 h, 78%; (i) p-TsOH (3.0 equiv), t-BuOH/CH2Cl2, 40 °C, 24 h, 59% + 19% recovered starting material. p-TSOH = para-toluenesulfonic acid, py = pyridine, DMAP = 4-dimethylaminopyridine.