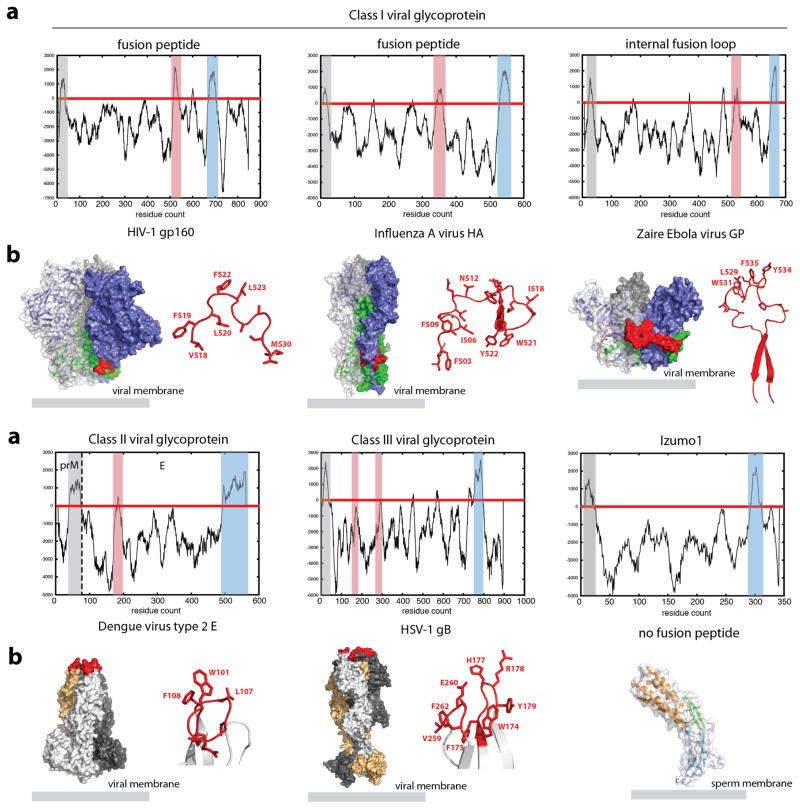
Extended Data Figure 8. Comparison of Izumo1 with selected viral fusogens.
A common feature of many viral fusogens is the presence of a hydrophobic fusion peptide or fusion loop. (a) A Kyte and Doolittle hydropathy plot was calculated for Izumo1, HIV-1 gp160, influenza A HA, Ebola virus GP, Dengue virus type 2 E, and herpes simplex virus-1 gB to detect the presence of hydrophobic regions. Class I and class II viral fusion glycoproteins (GPs) contain three clear hydrophobic regions corresponding to the signal peptide (highlighted in grey), fusion peptide or loop (highlighted in red) and the transmembrane anchor (highlighted in blue). For class III viral GPs, the presence of a signal peptide and transmembrane anchor are clear, however the hydrophobic fusion loop is formed by two discontinuous regions. This results in a lower hydropathy scale that is more difficult to detect. Two regions of hydrophobic residues cluster at the tip of the GP (shown in red) and are thought to be the internal fusion loop. In all class I, II and III viral fusion GPs, a clustering of aromatic and hydrophobic residues in a loop or helical region are hallmark features of the proteins for fusion. In contrast, Izumo1 clearly does not have any hydrophobic regions or structural features similar to the viral fusogens that could insert into the egg membrane. (b) Molecular surface representation of the class I, II, and III viral GPs and Izumo1. The fusion peptide/loop is shown as red sticks and also coloured red on the GP surface. For the class I viral GPs, the metastable prefusion trimer is shown, with the receptor binding and fusion subunits shown in blue and green, respectively. For the class II and class III viral GPs, the postfusion trimer is shown with three hydrophobic fusion loops clustered at the tip of the molecule.