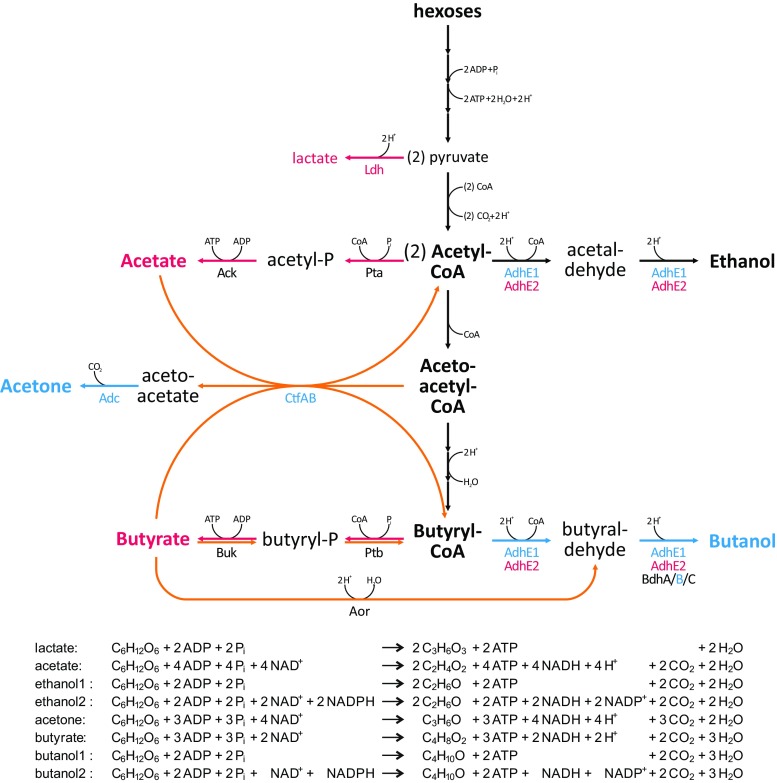
Fig. 2.
The metabolic network of ABE fermentation in wild-type C. acetobutylicum (Jones and Woods 1986). During acidogenesis (high pH), the culture predominantly forms the organic acids acetate and butyrate (red), whereas solventogenesis (low pH) features the formation of the solvents acetone and butanol (blue). Ethanol is formed in minor but similar amounts during both metabolic phases. In particular, solvent-forming enzymes are subject to state-dependent synthesis as indicated by the different colours. Three different mechanisms for acid re-assimilation (orange) are considered in the models discussed in this review: (1) acetate and butyrate cycles consisting of CtfAB-dependent acid assimilation and ATP-forming acid kinase reactions coupled to acetone formation (Hartmanis et al. 1984); (2) a reverse Buk-Ptb mechanism (Desai et al. 1999a); and (3) Aor-dependent re-utilization (Millat et al. 2014). The chemical equations below the network summarize the mass balances resulting from stoichiometric conversion of glucose to the indicated products. Two equations are given for the formation of alcohols since aldehyde/alcohol dehydrogenases (AdhE) use NADH as cofactor, whereas butanol dehydrogenases (Bdh) use NADPH. Note that the function of AdhE1 as alcohol dehydrogenase has been challenged (Yoo et al. 2015) (colour figure online)