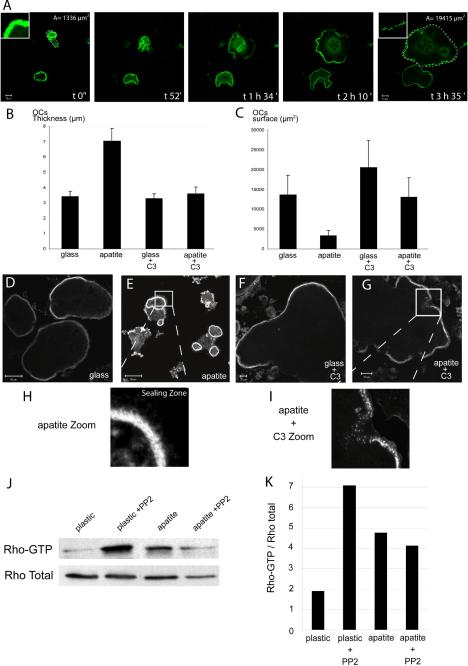
Figure 6.
Rho is required for sealing zone formation and polarization of osteoclast on apatite. Mature osteoclasts adherent on glass or apatite-coated slides were either untreated or treated with exoenzyme C3 (100 nM) for 5 h before being observed by confocal microscopy after actin staining. (A) Images of resorbing osteoclasts on apatite taken at different time point show the transition from polarized (1336 μm2 surface area) to largely spread cells (1945 μm2 at time 3 h 35 min) as well as the transition from sealing zone to podosome belt (insets). (B and C) Graphical representation of the thickness and surface area of osteoclasts, seeded either on glass or apatite and treated, or not, with C3. Mineral substratum increased osteoclast average thickness (B) and decreased surface area (C and E) compared with glass, (C and D) indicating that they were polarized (n = 30), as measured using Zeiss LSM 510 software. Exoenzyme C3–mediated Rho inhibition completely abrogated apatite-dependent sealing zone formation. In the presence of exoenzyme C3, osteoclasts were flat and spread (B, C, F, and G). Osteoclasts seeded on apatite, in the presence of C3, exhibited podosome belts (G and I) instead of the sealing zones seen in untreated cells (E and H). (J) Differentiated osteoclasts were seeded on plastic or apatite for 12 h, and Rho activity was assessed by pull-down binding assays. After 12 h on apatite, the level of Rho-GTP was clearly increased compared with plastic. In presence of PP2 after 12 h on plastic, the level of Rho-GTP increased, but was maintained on the apatite substratum. This experiment has been repeated three times with similar results, and one of them is shown here. (K) Rho GTP vs. total Rho ratio was evaluated by Western blotting and quantitated using Image Quant software.