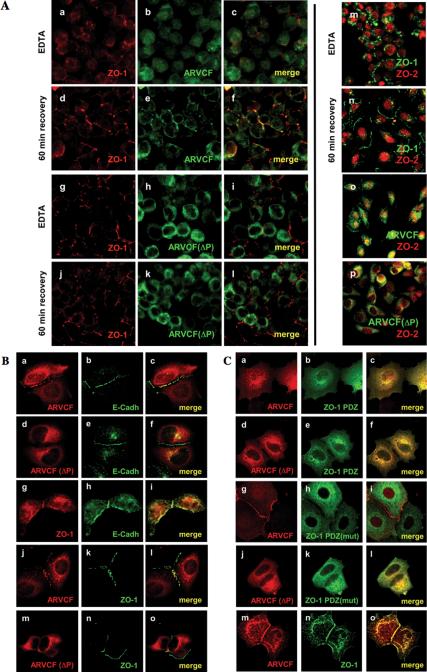
Figure 7.
Plasma membrane recruitment and nuclear localization of ARVCF require the PDZ-binding motif and are regulated by cell-cell adhesion. (A) Calcium switch experiments. MDCK cells grown on filters and expressing either ARVCF (a-f) or ARVCF(ΔP) (g-l) were incubated with EDTA to deplete calcium and disrupt cell-cell adhesion. The distribution of ARVCF (green) and ZO-1 (red) was analyzed after EDTA-treatment or after the incubation for 60 min in calcium-containing media to allow reestablishment of cell-cell adhesion, by staining with suitable antibodies and visualization by confocal microscopy. In panels where staining for ARVCF and ZO-1 was merged, yellow indicates colocalization of the two proteins. In m-p, ZO-2 (red) was colocalized with ZO-1 (green, m and n), or ARVCF or ARVCF(ΔP) (green, o and p, respectively), either after calcium depletion or a 60-min recovery. (B) Recruitment of ARVCF to sites of cell-cell contact in MCF7 cells. Sparsely plated MCF7 cells either transfected with cDNAs for ARVCF (a-c and j-l), ARVCF(ΔP) (d-f and m-o), or not transfected (g-i) were processed for immunofluorescence staining, and the localization of ARVCF, ARVCF(ΔP), and endogenous E-cadherin and ZO-1 visualized by confocal microscopy. Colocalization of the respective proteins is yellow in the merged images. (C) Effect of ZO-1 mutants on ARVCF localization in MCF7 cells. Sparse MCF7 cells coexpressing ARVCF or ARVCF(ΔP) (a-c, g-i and d-f, j-l, respectively) and either ZO-1 PDZ domains (a-f), a ZO-1 PDZ carrying inactivated PDZ-domains [PDZ(mut); g-l] or a full-length ZO-1 construct were analyzed by immunofluorescence staining and confocal microscopy. ZO-1 constructs carried an N-terminal myc tag. Colocalization of the respective proteins is yellow in the merged images.